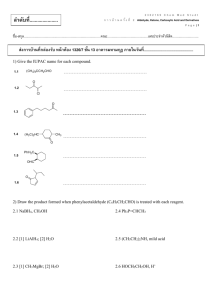
bip22510-sup-0002-suppinfo02
advertisement
SUPPORTING INFORMATION Structural similarity between 3-peptides synthesized from 3-homo-amino acids or L-aspartic acid monomers Sahar Ahmed,1, † Tara Sprules,2 Kamaljit Kaur1, * Table of Contents Table S1. Characterization of 3-peptides 2-6............................................................................. S2 Figure S1. RP-HPLC traces of four crude β3-amido amino acid monomers. ............................. S3 Figure S2. RP-HPLC chromatograms of pure β3-peptides 2-6. .................................................. S4 Table S2: 1H NMR chemical shift assignments for β3-peptide 2 in CF3CD2OH. ....................... S5 Table S3: 1H NMR chemical shift assignments for 3-peptide 3a in CF3CD2OH. ...................... S6 Table S4: 1H NMR chemical shift assignments for 3-peptide 3b in CF3CD2OH....................... S7 Table S5: 1H NMR chemical shift assignments for 3-peptide 4a in CF3CD2OH. ...................... S8 Table S6: 1H NMR chemical shift assignments for 3-peptide 4b in CF3CD2OH. ...................... S9 Table S7: 1H NMR chemical shift assignments for 3-peptide 5a in CF3CD2OH. .................... S10 Table S8: 1H NMR chemical shift assignments for 3-peptide 5b in CF3CD2OH..................... S11 Table S9: 1H NMR chemical shift assignments for 3-peptide 6 in CF3CD2OH. ...................... S12 Figure S3. Circular dichroism (CD) spectra of β3-peptides 3-5 (200 μM) in TFE. .................. S13 Figure S4. 2D NOESY of β3-peptides 2, 3a, and 6 in TFE. ...................................................... S15 Table S10. Structure calculation statistics for β3-hexapeptide 2. .............................................. S16 Table S11. Structure calculation statistics for β3-hexapeptide 6. .............................................. S16 Figure S5. Solution structures of -peptides 1 and 2. .............................................................. S17 S1 Table S1. Characterization of 3-peptides 2-6. Peptide Mass [M+H]+ Obsd. (Calcd.) HPLC Gradient 2 782.9 (782.6) 20-65% ACN/H2O 16.0 2 89 3a 699.8 (699.4) 12-20% ACN/H2O 29.5 2 62 3b 685.3 (685.4) 12-20% ACN/H2O 30.0 2 53 4a 885.8 (885.5) 12-45% ACN/H2O 21.0 2 72 4b 871.8 (871.4) 19-28% ACN/H2O 22.0 2 54 5a 1041.6 (1041.2) 19-28% IPA/H2O 23.0 1.5 81 5b 1027.9 (1027.6) 19-40% ACN/H2O 21.5 2 82 Elution Flow rate time (ml/min) (min) Yield (%) 1100.4 (1100.6) 15-30% IPA/H2O 16.7 1.5 90 6 Vydac C18 semi-preparative (1 x 25 cm, 5 μm) HPLC column was used. A gradient of ACN/water or IPA/water in 30 minutes with a flow rate of 1-5-2.0 mL/min was used. S2 (a) O Absorbance (mV) Fmoc (c) (b) NH O COOH N H Fmoc NH COOH N H (d) O O O O HN O O Fmoc N H NH Fmoc COOH N H NH COOH Time (Min) Figure S1. RP-HPLC traces of four crude β3-amido amino acid monomers, Fmoc-β3amV-OH (a), Fmoc-β3amL-OH (b), Fmoc-β3amK(Boc)-OH (c) and Fmoc-β3amE(tBu)-OH (d). The HPLC runs were performed using 30-100% IPA/H2O in 35 min with flow rate, 2 mL/min (insert shows the structure of each monomer). S3 Absorbance (mV) Time (Min) Figure S2. RP-HPLC chromatograms of pure β3-peptides 2-6 obtained using analytical Vydac C18 analytical column (0.46 x 25 cm, 5 μm). S4 Table 02: 1H NMR chemical shift assignments for β3-peptide 2 in CF3CD2OH (500 MHz). Residue Backbone shifts (δ) 1 2 3 4 5 7.39 (1H, H3N+), 3.57(1H,NHCHCH2), 2.89,2.68(2H,NHCHCH2) 8.34(d,J=9.4Hz,HN), 4.52(1H,NHCHCH2), 2.75,2.57(2H,NHCHCH2), 8.27(d,J=9.2Hz,HN), 4.36(1H,NHCHCH2), 2.46(2H,NHCHCH2) 7.47(d,J=9.4Hz,HN), 4.24(1H,NHCHCH2), 2.57,2.26(2H,NHCHCH2), 6.76 (d, J=8.9 Hz,HN), 4.38(1H,NHCHCH2), 2.53,2.34(2H,NHCHCH2), 6.54(d,J=9.5Hz,HN), 4.45(1H,NHCHCH2), 2.50,2.30(2H,NHCHCH2), 6.85, 5.82 (NH2) C-terminus 6 Side chain shifts(δ) 2.09(1H,(CH3)2CH), 1.09(6H,(CH3)2CH) 7.29(3H,NH3+CH2CH2CH2CH2), 3.03,2.97(2H,NH3CH2CH2CH2CH2), 1.79,1.68(2H,NH3CH2CH2CH2CH2), 1.58(2H,NH3CH2CH2CH2CH2), 1.45,1.42(2H,NH3CH2CH2CH2CH2) 1.52(1H,(CH3)2CHCH2), 1.45, 1.28(2H,(CH3)2CHCH2), 0.94,0.89(6H,(CH3)2CH CH2) 1.73(1H,(CH3)2CH), 0.91(6H,(CH3)2CH) 7.31(3H,NH3+CH2CH2CH2CH2), 2.97(2H,NH3CH2CH2CH2CH2), 1.68(2H,NH3CH2CH2CH2CH2), 1.58,1.45(2H,NH3CH2CH2CH2CH2), 1.38(2H,NH3CH2CH2CH2CH2) 1.58(1H,(CH3)2CHCH2), 1.42, 1.31(2H,(CH3)2CHCH2), 0.94(6H,(CH3)2CH CH2) The final conc. of 2 in TFE-d2 was 5 mM. The chemical shifts were referenced to the TFE methylene protons at 3.88 ppm and recorded at 15 ºC. S5 Table 03: 1H NMR chemical shift assignments for 3-peptide 3a in CF3CD2OH (500 MHz). Residue Backbone shifts (δ) 1 2 3 4 7.77 (NH3+-terminal), 4.28 (1H,NHCHCH2), 3.08, 2.88 (2H,NHCHCH2), 8.21 (1H, HN), 4.88 (1H,NHCHCH2), 2.75, 2.64(2H,NHCHCH2) 7.63 (1H, HN), 4.79(1H,NHCHCH2), 2.80, 2.66(2H,NHCHCH2), 7.54 (1H, HN), 4.93 (1H,NHCHCH2), 2.77,2.67(2H,NHCHCH2), Side chain shifts (δ) 7.57 (1H, NH). 3.04 , 3.18 (2H, (CH3)2CHCH2), 1.78 (1H, (CH3)2CH), 0.90(6H, (CH3)2CHCH2), 7.02 (1H, NH) 3.96 (1H, (CH3)2CH), 1.15 (6H, (CH3)2CH), 7.29 (1H, NH), 7.20 (3H, NH3+) 3.25 (2H,NH3CH2CH2CH2CH2), 3.00(2H,NH3CH2CH2CH2CH2), 1.66 (2H,NH3CH2CH2CH2CH2), 1.57 (2H,NH3CH2CH2CH2CH2), 7.68 (1H, NH), 3.12 (1H (CH3)2CH CH2), 3.00 (1H, (CH3)2CH CH2), 1.80 (1H,(CH3)2CH), 0.92 (6H, (CH3)2CHCH2), 6.85, 6.52 (NH2) C- terminus 3 The final conc. of -peptide 3a in TFE-d2 was 0.5 mg/250 μL. The chemical shifts were referenced to the TFE methylene protons at 3.88 ppm. NH2 terminal of residue 1 was not completely assigned from NMR. S6 Table 04: 1H NMR chemical shift assignments for 3-peptide 3b in CF3CD2OH (500 MHz). Residue Backbone shifts (δ) 1 2 3 4 Side chain shifts (δ) 7.71 (NH3+-terminal ), 4.27 (1H,NHCHCH2), 3.20, 2.99 (2H,NHCHCH2), 8.17 (1H, HN), 4.88 (1H,NHCHCH2), 2.93(2H,NHCHCH2) 7.61 (1H, HN), 4.82(1H,NHCHCH2), 2.89 (2H,NHCHCH2) 7.57 (1H, HN), 4.93 (1H,NHCHCH2), 2.77, 2.60(1H,NHCHCH2) C- terminus 7.57 (1H, NH). 3.20, 2.99 (2H (CH3)2CHCH2), 1.8 (1H, (CH3)2CH), 0.91 (6H, (CH3)2CHCH2), 7.03 (1H, NH) 4.02 (1H, (CH3)2CH), 1.16 (6H, (CH3)2CH) 3.45- 3.33(2H,NH3CH2CH2CH2), 2.60-2.77 (4H,NH3CH2CH2CH2) 7.69 (1H, NH) 2.60-2.77 (2H (CH3)2CHCH2), 1.80 (1H,(CH3)2CH), 0.91 (6H, (CH3)2CHCH2), 6.86, 6.48 (NH2) The final conc. of 3-peptide 3b in TFE-d2 was 0.5 mg/250 μL. The N-terminal NH3+ was not completely assigned due to exchange with the solvent. S7 Table 05: 1H NMR chemical shift assignments for 3-peptide 4a in CF3CD2OH (500 MHz). Residue Backbone shifts (δ) Side chain shifts (δ) 1 4.27 (1H,NHCHCH2), 2.643.14 (2H,NHCHCH2) 2 8.57(1H, HN), 4.80 (1H,NHCHCH2), 2.64-3.14 (2H,NHCHCH2), 3 8.57 (1H, HN), 5.00 (1H,NHCHCH2), 2.64-3.14 (2H,NHCHCH2) 7.31 (1H, HN), 5.05 (1H,NHCHCH2), 2.64-3.14 (2H,NHCHCH2) 6.74 (1H, NH) 4.02 (1H, (CH3)2CH), 1.14 (6H, (CH3)2CH) 7.29 (1H, HN), 4.27 (1H,NHCHCH2), 2.56 (2H,NHCHCH2) 8.34 (1H, NH), 3.32(2H,(CH3)2CHCH2), 1.78(1H, (CH3)2CH), 0.91 (6H, (CH3)2CH) 4 5 C- terminus 7.79(NH), 3.72(1H,COOHCH2CH2NH) 3.64(1H,COOHCH2CH2NH), 2.54 (2H COOHCH2CH2NH) 7.24 (1H, NH), 3.44(2H,(CH3)2CHCH2), 1.80 (1H, (CH3)2CH), 0.91 (6H, (CH3)2CH) 7.96 (1H, NH), 7.20 (3H, NH3+) 3.47 (2H,NH3CH2CH2CH2CH2), 1.68-1.84 (2H,NH3CH2CH2CH2CH2) 6.76, 6.58 (NH2) The final conc. of β3-peptide 4a in TFE-d2 was 0.6 mg/250 μL. The N-terminal NH3+ was not observed due to exchange with the solvent. One of the CH2 protons of residue 4 were not assigned from the NMR due to broadness of the peaks. S8 Table 06: 1H NMR chemical shift assignments for 3-peptide 4b in CF3CD2OH (500 MHz). Residue Backbone shifts (δ) 1 4.26 (1H,NHCHCH2), 3.36,2.84 (2H,NHCHCH2) 2 8.50 (1H, HN), 4.80 (1H,NHCHCH2), 2.71,2.61 (2H,NHCHCH2) 3 8.50 (1H,HN), 5.00 (1H,NHCHCH2), 2.90,2.64 (2H,NHCHCH2) 7.44 (1H,HN), 5.03 (1H,NHCHCH2), 2.78,2.68 (2H,NHCHCH2) 4 Side chain shifts (δ) 7.78(NH),3.63(1H,COOHCH2CH2NH) 3.47(1H,COOHCH2CH2NH), 2.54 (2H COOHCH2CH2NH) 7.27 (1H, NH), 3.05(2H,(CH3)2CHCH2), 1.77(1H, (CH3)2CH), 0.89 (6H, (CH3)2CH CH2) 6.75 (1H, NH) 3.96 (1H, (CH3)2CH), 1.13 (6H, (CH3)2CH) 8.12 (1H, NH), 7.20 (3H, NH3+) 3.38 (2H,NH3CH2CH2CH2), 3.03 (2H,NH3CH2CH2CH2), 1.95 (2H,NH3CH2CH2CH2), 7.28 (1H, NH), 4.26 8.25 (1H, NH), 3.11, 2.96(2H,(CH3)2CHCH2), 1.81(1H, (1H,NHCHCH2), 2.72-2.64 (CH3)2CH), 0.91 (6H, (CH3)2CH) (2H,NHCHCH2) C- terminus 6.76, 6.56 (NH2) 5 The final conc. of β3-peptide 4b in TFE-d2 was 0.6 mg/250 μL. The N-terminal NH3+ was not observed due to exchange with the solvent. S9 Table 07: 1H NMR chemical shift assignments for 3-peptide 5a in CF3CD2OH (500 MHz). Residue Backbone shifts (δ) 1 2 3 4 5 6 Side chain shifts (δ) 5.15 (1H,NHCHCH2), 2.93,2.70 (2H,NHCHCH2) 8.89 (1H, (HN-terminal), 5.00 (1H,NHCHCH2), 3.16,2.57 (2H,NHCHCH2) 9.11 (1H, HN), 4.79 (1H,NHCHCH2), 3.06,2.57 (2H,NHCHCH2) 7.01 (1H, NH) 4.02 (1H, (CH3)2CH), 1.14 (6H, (CH3)2CH) 7.56(NH), 3.52,3.44 (1H,COOHCH2CH2NH) 2.50 (2H COOHCH2CH2NH) 8.50 (1H, HN), 5.08 (1H,NHCHCH2), 2.64,2.47 (2H,NHCHCH2) 7.30 (1H, HN), 5.30 (1H,NHCHCH2), 2.68 (2H,NHCHCH2) 7.75 (1H, NH) 4.00 (1H, (CH3)2CH), 1.20 (6H, (CH3)2CH) 7.19 (1H, HN) 5.22 (1H,NHCHCH2), 2.70 (2H,NHCHCH2) 8.20 (1H, NH), 3.06, 3.02(2H,(CH3)2CHCH2), 1.80(1H, (CH3)2CHCH2), 0.89 (6H, (CH3)2CH CH2) C- terminus 6.93 (1H, NH), 3.06, 3.02(2H,(CH3)2CHCH2), 1.77(1H, (CH3)2CH CH2), 0.87 (6H, (CH3)2CH CH2) 8.43 (1H, NH), 3.44,3.06(2H,NH3CH2CH2CH2CH2) 3.02(2H,NH3 CH2CH2CH2CH2), 1.63(2H,NH3CH2CH2CH2CH2), 1.70(2H,NH3CH2CH2CH2 CH2) 6.88, 6.73 (NH2) The final conc. of 3-peptide 5a in TFE-d2 was 0.2 mg/250 μL. The N-terminal and side chain NH3+ were not observed due to exchange with the solvent. S10 Table 08: 1H NMR chemical shift assignments for 3-peptide 5b in CF3CD2OH (500 MHz). Residue Backbone shifts (δ) 1 2 3 4 5 2.93, 2.71 (2H,NHCHCH2) 8.81 (1H, (HN-terminal), 5.03 (1H,NHCHCH2), 3.09, 2.60 (2H,NHCHCH2) 9.01 (1H, HN), 4.81 (1H,NHCHCH2), 3.08, 2.60 (2H,NHCHCH2) 8.47 (1H, HN), 5.11 (1H,NHCHCH2), 2.65, 2.53 (2H,NHCHCH2) 7.46 (1H, HN), 2.71 (2H,NHCHCH2) Side chain shifts (δ) 7.03 (1H, NH), 4.05 (1H, (CH3)2CH),1.15 (6H, (CH3)2CH) 7.62(NH), 2.53(2H COOHCH2CH2NH) 3.55,3.47(2H COOHCH2CH2NH) 6.97 (1H, NH),3.07, 3.04(2H, (CH3)2CH CH2), 1.78(1H, (CH3)2CH CH2), 0.90 (6H, (CH3)2CH CH2) 7.74 (1H, NH)1.18 (6H, (CH3)2CH) 4.05 (1H, (CH3)2CH), 8.52 (1H, NH), 3.46, 3.30 (2H,NH3CH2CH2CH2) 3.03 (2H,NH3CH2CH2CH2) 1.96 (2H,NH3CH2CH2CH2) 7.25 (1H, HN) 5.22 8.16(1H,NH),3.07,2.99 (1H,NHCHCH2), 2.72 (2H,(CH3)2CHCH2)1.80(1H,(CH3)2CHCH2),0.90 (6H, (2H,NHCHCH2) (CH3)2CH CH2) C- terminus 6.85, 6.76 (NH2) The final conc. of 3-peptide 5b in TFE-d2 was 0.6 mg/250 μL. The N-terminal and side chain NH3+ were not observed due to exchange with the solvent. 6 S11 Table 09: 1H NMR chemical shift assignments for 3-peptide 6 in CF3CD2OH (500 MHz). Residue Backbone shifts (δ) Side chain shifts (δ) 1 4.34 (1H,NHCHCH2), 3.49,2.89(2H,NHCHCH2) 2 8.67(d, J=9.0Hz, HN), 5.06 (1H,NHCHCH2), 3.182.58(2H,NHCHCH2) 3 9.08(d, J-8Hz, HN), 4.85 (1H,NHCHCH2), 3.09,2.60(2H,NHCHCH2) 6.94 (1H, NH), 3.07(2H,(CH3)2CHCH2), 1.77(1H, (CH3)2CH), 0.89 (6H, (CH3)2CH) 4 8.52(d, J=7.9 Hz, HN), 5.03 (1H,NHCHCH2), 2.75,2.67(2H,NHCHCH2) 5 7.28 (1H, HN), 5.28(1H,NHCHCH2), 2.74,2.67(2H,NHCHCH2) 6 7.22(1H, HN), 5.23 (1H,NHCHCH2), 2.77(2H, NHCHCH2). 8.08(1H, NH),7.30(3H,NH3+) 3.33(2H,NH3CH2CH2CH2CH2), 3.03(2H,NH3CH2CH2CH2CH2), 1.73(2H,NH3CH2CH2CH2CH2), 1.64 (2H,NH3CH2CH2CH2CH2) 8.39 (1H, NH),7.24(3H,NH3+) 3.33(2H,NH3CH2CH2CH2CH2), 3.03(2H,NH3CH2CH2CH2CH2), 1.72(2H,NH3CH2CH2CH2CH2), 1.64 (2H,NH3CH2CH2CH2CH2), 8.23 (1H, NH) 3.07, 2.99(2H (CH3)2CH CH2), 1.82 (1H, (CH3)2CH), 0.91 (6H, (CH3)2CHCH2), C- terminus 7.79(NH), 3.66(1H,COOHCH2CH2NH) 3.49(1H,COOHCH2CH2NH), 2.58 (2H COOHCH2CH2NH) 7.57(NH), 3.56,(1H,COOHCH2CH2NH) 3.45(1H,COOHCH2CH2NH), 2.56 (2H COOHCH2CH2NH) 6.78, 6.72 (NH2) The final conc. of 3-peptide 6 in TFE-d2 was 1.5 mg/250 μL. The N-terminal NH3+ was not observed due to exchange with the solvent. S12 0 2 -1 x 10 (deg cm dmol ) (a) -10 -3 5a 4a 3a -20 210 225 240 (b) -1 x 10 (deg cm dmol ) 195 2 0 -10 -3 5b 4b 3b -20 195 210 225 240 Wavelength (nm) Figure S3. Circular dichroism (CD) spectra of β3-peptides 3-5 (200 μM) in TFE. S13 (a) NH2 H H2N O H N H O NH2 HO H O N H N H HO N H H NH2 N H 1H-4H 2H-5H 3H-6H 2 S14 O (b) NH2 NH2 COOH O O NH O O H NH O O H NH O O H H2N N H N H N H NHO H NH2 H2N NH O O H N H NH2 COOH NH O O H N H NH O O H NH O O H N H N H NH O O H NHO H N H 1H-4H 1H-4H 2H-5H 3H-6H 6 3a Figure S4. 2D NOESY of β3-peptides 2, 3a, and 6 in TFE showing the long range NOEs, CH(i)-CH(i+3) characteristic of 14-helix conformation. S15 NH2 Table S10. Structure calculation statistics for β3-hexapeptide 2. NOE upper distance limits 170 Intra-residue 68 Sequential 47 Medium range (I to i-2 or i+3) 53 Long range (I to i+4) 2 a Final CYANA structures CYANA target function 0.011±0.008 Average backbone RMSD 0.54±0.10 Å Average heavy atom RMSD to mean 1.15±0.15 Å Distance restraint violations 0 a 20 lowest energy structures of the 200 calculated. Table S11. Structure calculation statistics for β3-hexapeptide 6. NOE upper distance limits 167 Intra-residue 71 Sequential 42 Medium range (I to i-2 or i+3) 53 Long range (I to i+4) 1 a Final CYANA structures CYANA target function 0.0298±0.0287 Average backbone RMSD 0.50±0.12 Å Average heavy atom RMSD to mean 1.44±0.14 Å Distance restraint violations 0 a 20 lowest energy structures of the 200 calculated. S16 Figure S5. Solution structures of -peptides 1 and 2 showing comparison of the right-handed and the left-handed helices, respectively. S17