Precipitation reactions
advertisement

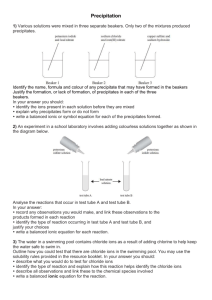
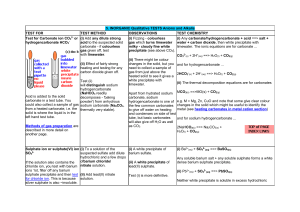
Precipitate Reactions L.O. • I can state which are spectator ions in a precipitation reaction. • I can make a clean dry precipitate and write the ionic equation for the formation of the precipitate Precipitation reactions An insoluble solid that forms during an aqueous reaction is called a precipitate. A reaction which forms a precipitate is called a precipitation reaction. The limewater test for carbon dioxide is a precipitation reaction. Limewater is actually a dilute solution of calcium hydroxide. The calcium hydroxide reacts with carbon dioxide to form calcium carbonate, which is insoluble in water: calcium carbon + hydroxide dioxide Ca(OH)2 + CO2 calcium + water carbonate CaCO3 + H2O Uses of precipitation reactions Most precipitation reactions are very fast reactions that occur between ions. This makes them very useful for identifying specific ions based on the type of precipitate formed. Precipitation reactions have a number of other uses: production of coloured pigments for paints and dyes removal of toxic chemicals from water separation of reaction products. A lead iodide precipitate. Isolating the precipitate The precipitate from a precipitation reaction can be separated from the reaction mixture by filtration. Buchner funnel vacuum pump A Buchner funnel and flask can be used to accelerate the process. filter paper This apparatus uses a vacuum pump to draw the mixture through the filter. Buchner flask The filtrate is finally washed and dried. Spectator ions In ionic precipitation reactions there are often ions that are not involved in the reaction. These are known as spectator ions. NaI (aq) + AgNO3 (aq) AgI (s) + NaNO3 (aq) The spectator ions are easily identified using the ionic equation. Na+ (aq) + I– (aq) + Ag+ (aq) + NO3– (aq) AgI (s) + Na+ (aq) + NO3– (aq) This equation shows that the silver and the iodine ions have reacted, joining together to make the precipitate. The sodium (Na+) and nitrate (NO3–) ions are spectator ions. This means the ionic equation can be simplified to: I– (aq) + Ag+ (aq) AgI (s) Which ions are spectators? Silver halides The different silver halide precipitates can be distinguished by their differing colours. chloride bromide iodide white AgCl precipitate cream AgBr precipitate yellow AgI precipitate Identifying negative ions: halides Halide ions are formed from the Group VII elements, the halogens. Halides are detected using silver nitrate solution. The substance to be tested is first acidified with a small amount of nitric acid before adding the silver nitrate solution. If halides are present, a precipitate will form. The precipitates formed are silver halides: sodium + chloride silver nitrate NaCl (aq) + AgNO3 (aq) Cl– (aq) + Ag+ (aq) silver + chloride sodium nitrate AgCl (s) + NaNO3 (aq) AgCl (s) Identifying negative ions: sulfate Sulfate ions (SO42–) are identified by adding a few drops of barium chloride solution. The solution must be acidified first with a few drops of hydrochloric acid. A white precipitate of barium sulfate forms. sodium sulfate Na2SO4 (aq) + barium chloride + BaCl2 (aq) barium sulfate BaSO4 (s) + 2NaCl (aq) The ionic equation for this reaction is: SO42– (aq) + Ba2+ (aq) sodium + chloride BaSO4 (s) Method Add the chemicals together and write the symbol equations for each one, remember to include state symbols copper sulphate CuSO4 and sodium hydroxide NaOH iron II chloride FeCl2 and sodium hydroxide NaOH iron III chloride FeCl3 and sodium hydroxide NaOH potassium chloride KCl and silver nitrate AgNO3 potassium bromide KBr and silver nitrate AgNO3 potassium iodide KI and silver nitrate AgNO3 potassium sulphate KSO4 and barium chloride BaCl2 lead nitrate Pb(N03)2 and potassium iodide KI Finish symbol equation – balance – add state symbols Equations CuSO4(aq) + 2NaOH (aq) FeCl2 + 2NaOH (aq) (aq) Cu(OH)2 + Na2SO4 (aq) (s) Fe(OH)2 (s)+ 2 NaCl (aq) FeCl3(aq)+ 3NaOH(aq) Fe(OH)3(s)+ 3 NaCl (aq) KCl (aq)+ AgNO3 KNO3(aq) + AgCl (s) (aq) Finish symbol equation – balance – add state symbols Equations KBr (aq) + AgNO3(aq) KI (aq) + AgNO3(aq) KNO3 + AgBr(s) KNO3(aq)+ AgI (aq) (s) K2SO4 + BaCl2 2 KCl(aq)+ BaSO4 (s) Pb(NO3)2 + 2 KI 2KNO3(aq) + PbI2 (s) (aq) (aq) (aq) (aq) State symbols