Atoms and Elements
advertisement

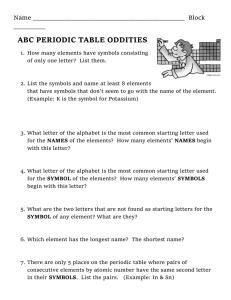
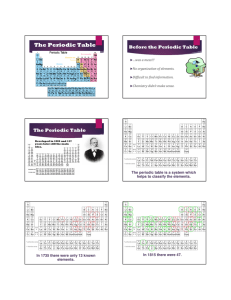
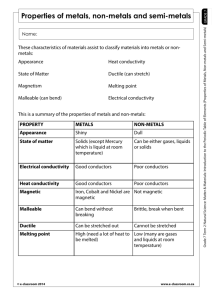
Score Earned Name:_____________________ Partner:___________________ Atoms and Elements Core:_________ Date:_________ Directions: Read through each page of the website provided completely. To answer the following questions, read about each topic and use complete and thoughtful sentences. Everyone must turn in their own work. Atoms 1. Atoms are incredibly tiny. Can you see them with a microscope? _________________________________________________________ Elements 2. Elements are unique types of atoms. Give at least three examples of elements 1. ___________________________ 2. ___________________________ 3. ___________________________ 4. ___________________________ 5. ___________________________ 3. What does it mean when we refer to gold as “pure gold”? _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ 4. Chemical reactions split molecules and can rearrange atoms. Can a chemical reaction change one element, like silver, into another element, like gold? Explain your answer. _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ Chemical Symbols 5. Each element is given its own chemical symbol. Give three examples of chemical symbols and the elements they stand for Symbol Element 1. _______ ___________________ 2. _______ ___________________ 3. _______ ___________________ 6. Which is the correct way to write the chemical symbol for iron? fe fE Fe FE Based on what you learned about chemical symbols, why was this the best answer? _________________________________________________________ _________________________________________________________ _________________________________________________________ 7. Chemical symbols are not always based on the name of an element. Give two examples of elements that have chemical symbols that are not from the name of the element Symbol Element 1. _______ ___________________ 2. _______ ___________________ The periodic table 8. In your own words, describe the periodic table of elements. _________________________________________________________ _________________________________________________________ _________________________________________________________ 9. Elements found in a row (left to right) are called a ___________________ Elements found in a column (up and down) are called a _________________ 10. Elements on the Periodic Table are sorted into what types of elements? Which category has more elements than the other? _________________________________________________________ _________________________________________________________ _________________________________________________________ 11. Which element is sometimes sorted separately from the rest? _________________________________________________________ 12. Steph is looking for water (H2O) on the periodic table, why won’t she be able to find it? _________________________________________________________ _________________________________________________________ _________________________________________________________ Metals 13. Metals are known to be malleable. What does the word malleable mean? _________________________________________________________ _________________________________________________________ 14. All metals are solid, except for one unique metallic element. Which element is liquid at room temperature? __________________________________ 15. Some metals are magnetic. Which three metals are magnetic? 1. ___________________________ 2. ___________________________ 3. ___________________________ 16. Steel is not an element, but it can be magnetic. Explain why this is true. _________________________________________________________ _________________________________________________________ Non-metals 17. How many non-metals are gases at room temperature?_________________ 18. How many non-metals are liquid at room temperature, and include their name(s). _________________________________________________________ 19. What does the graphite in your pencil have in common with a diamond? _________________________________________________________ _________________________________________________________ How are they different? ______________________________________ _________________________________________________________ _________________________________________________________ 20. Why is graphite unusual for a non-metal? __________________________ _________________________________________________________ _________________________________________________________ Complete the following table: Property Appearance State at room temperature Density Strength Malleable or brittle Conduction of heat Conduction of electricity Magnetic material Metals Non-metals