Metal vs non-metal
advertisement
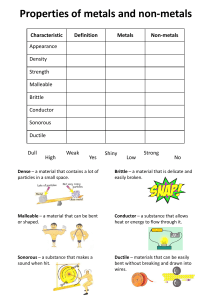
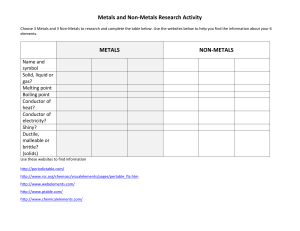
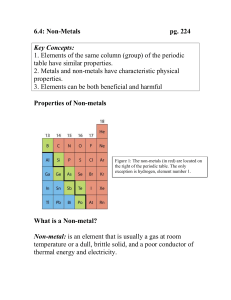
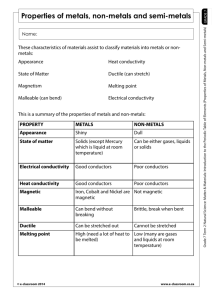
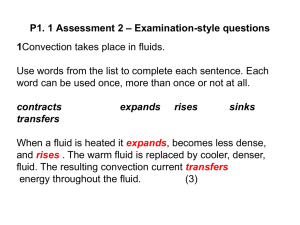
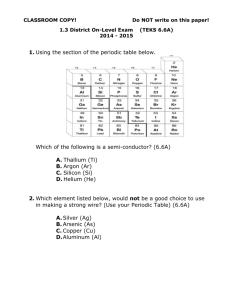
metals Metals are found on the _____________ of the periodic table. There are __________ metals than non-metals Common properties of metals include: Malleable: this means they can be bent and bashed into shape Ductile: this means they can be drawn out into thin wires Conductors: this means they allow heat and electricity to travel along them Shiney: they are reflective of light Usually grey: most metals are a grey colour Solid at room temperature: except mercury (liquid) Non-metals Common properties of non-metals include: Brittle: this means they break easily if hit Various colours: non-metals come in many colours Insulators: most non-metals are non-conductors, an exception is graphite (C), it is an excellent conductor of electricity. Solids, liquids and gases: non-metals come in all different states. Bromine (Br) is the only naturally occurring liquid non-metal. Problems: 1 Are there more metals or non-metals? _________________________________________ 2 Which side of the periodic table has the metals? _________________________________ 3 What is the atomic number of the following elements: sodium _____; copper _____; hydrogen _____; zinc _____; Radium _____; Xenon _____; mercury _____; He _____ 4 Which element on the periodic table is a yellow solid non-metal? ____________________ Encarta Answer each one as you go, we will do this as a class by looking up the information together 1. What are the physical properties of metals? 2. Which elements are considered to have both metal and non-metal properties? these are also known as semi-metals. 3. What are some of the chemical properties/reactions of metals (list at least 3 different reactions)? 4. Type in the word non-metals: What are some of the properties of non-metals? Stick in worksheet and complete it. Finish off the other worksheet you were given at the end of last term. Yellow book: pg pg 20-21 No yellow book: copy and complete the following problems. 1 complete the sentences below using these words: lustre, ductile, state, malleable, brittle A. a material which is easily hammered or pressed into shape is said to be: ___ B. a material which is shiny and reflects light well has a high: ___ C. a material which shatters or snaps easily is: ___ D. a material which can be pulled into a wire is : ___ E. at room temperature, all metals except for Hg (_________) are in the solid: ___ 2 after reading the descriptions below, decide if they are a metal or nonmetal A. a shiny, silvery solid which is brittle and does not conduct heat or electricity B. A soft, malleable grey substance, shiny when freshly cut. It conducts heat and electricity and has a low melting point. C. Shiny, conducts electricity when solid, brittle, very high melting point. D. A shiny, silvery liquid, conducts heat and electricity well. Malleable when solid E. Soft, malleable substance, low melting point. Doesn’t conduct heat or electricity