Unit 8 Test Review Answers
advertisement

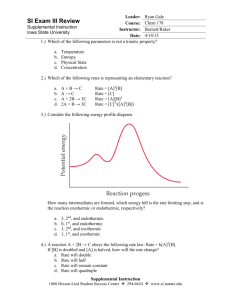
Unit 8 Test Review: Thermodynamics and Kinetics Thermodymics 1. Define the following terms. a. Heat: transfer of energy due to a temperature difference b. Temperature: measure of the average kinetic energy of the molecules of a substance c. Endothermic: heat is absorbed and taken in d. Exothermic: heat is released or exits the system e. Activation energy: the amount of energy needed by reactants in order to create products 2. Determine if the focus in each situation is endothermic or exothermic and how do you know? a. It’s been really cold outside lately. Johnny is set to go see his favorite team, the Georgia Bulldogs play a game tonight but it’s going to be 25°C. He decides to use some of his dad’s instant hand warmers to stay warm while he’s at the game. i. If we are focusing on instant hand warmers, are they endothermic or exothermic? The hand warmers are exothermic, they are giving off heat. ii. If we are focusing on Johnny’s hands, are they endothermic or exothermic? Johnny’s hands are endothermic, they are absorbing the heat. b. When you sprain your ankle, the nurse will give you an instant cold pack to reduce the swelling. Inside the cold pack is an inner bag and an outer bag. The inner bag contains water and the outer bag contains ammonium nitrate, NH4NO3. When you squeeze the cold pack, the inner bag breaks and the water reacts with the ammonium nitrate and the pack gets very cold. i. Is the reaction between water and ammonium nitrate endothermic or exothermic? The reaction is endothermic. ii. When you place the cold pack against your skin, is your skin endothermic or exothermic? Your skin is exothermic 3. Solve these heat problems using q = mCΔT. a. How much heat is released when 88.8g of calcium is cooled from 16°C to 10°C? The heat capacity of calcium is 0.650J/g°C. q = (88.8g)(0.650J/g°C)(-6°C) = -342.32J b. How much heat is absorbed when 42.6g of brass is heated from 25°C to 40°C? The heat capacity of brass is 0.380J/g°C. q = (42.6g)(0.380J/g°C)(15°C) = 242.82J c. What is the mass of lithium when 6570.6 J of heat is released when it cools from 30°C to 18°C? The heat capacity of lithium is 3.56J/g°C. m = -6570.6J/[(3.56J/g°C)(-12°C)] = 153.8g d. What is the change in temperature when -340000. J of energy is released when a ten pound lawn chair (4535.9 g) is left out overnight in a snow storm in Buffalo, New York. The heat capacity of aluminum is 0.902 J/g°C. ΔT = -340000J/[(4535.9g)(0.902J/g°C)] = -8.3°C Kinetics 4. Define the following terms: a. Catalyst: a substance that speeds up a reaction without being used up in the reaction. It lowers the activation energy needed for the reaction to proceed. b. Entropy: the degree of randomness or chaos in a system. c. 2nd Law of thermodynamics: processes have a tendency to move towards entropy 5. What are the 5 factors that can speed up the rate of a reaction and how do they do that? 1. Stirring: moves reactant particles by each other so the chance that they collide with the right amount of energy and in the right orientation increases, therefore, the reaction occurs faster. 2. Increasing temperature: causes the reactant particles to move faster by increasing their kinetic energy. The faster they move, the more often the particles collide which speeds up the reaction. 3. Increasing the concentration: this means that there are more reactant particles present to collide with, hence the reaction proceeds faster. 4. Increasing pressure (gases only): increasing thepressure causes the reactant partticles to come closer together which makes them more likely to collide and react faster. 5. Adding a catalyst: lowers the activation energy needed for the reaction to proceed. Stoichiometry Old Stuff 6. 2C4H10 + 13 O2 → 8CO2 + 10H2O a. How many moles of carbon dioxide are produced from the reaction of 26 moles of oxygen with excess butane (C4H10)? 26 moles O2 (8 mol CO2/13 mol O2) = 16 mol CO2 b. How many moles of water are produced from the reaction of 0.5 moles of butane with excess oxygen? 0.5 mol C4H10 (10 mol H2O/2 mol C4H10) = 2.5 mol H2O c. How many grams of water are produced from the reaction of 32.00g of oxygen with excess butane? 32.00g O2(1mol O2/32.00g O2)(10 mol H2O/13 mol O2)(18.02g H2O/1mol H2O) = 13.9g H2O