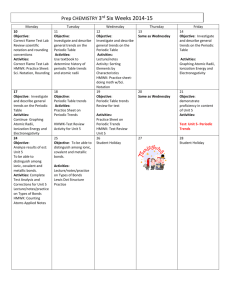
Prep CHEMISTRY 3rd Six Weeks 2014
advertisement
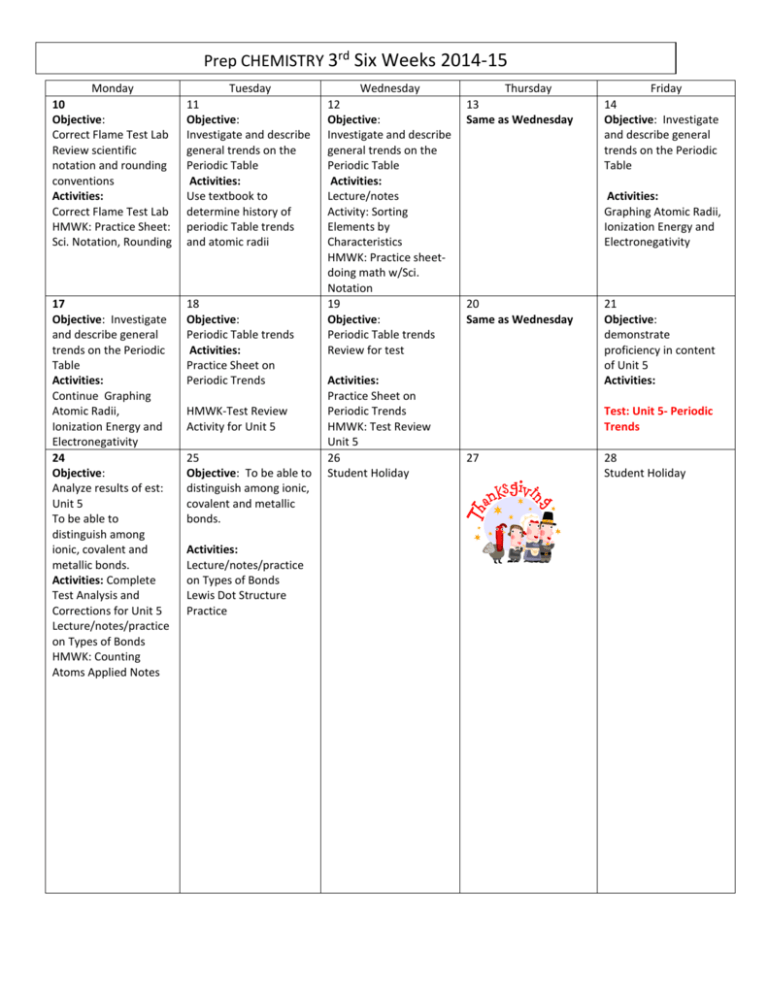
Prep CHEMISTRY 3rd Six Weeks 2014-15 Monday 10 Objective: Correct Flame Test Lab Review scientific notation and rounding conventions Activities: Correct Flame Test Lab HMWK: Practice Sheet: Sci. Notation, Rounding Tuesday 11 Objective: Investigate and describe general trends on the Periodic Table Activities: Use textbook to determine history of periodic Table trends and atomic radii 17 Objective: Investigate and describe general trends on the Periodic Table Activities: Continue Graphing Atomic Radii, Ionization Energy and Electronegativity 24 Objective: Analyze results of est: Unit 5 To be able to distinguish among ionic, covalent and metallic bonds. Activities: Complete Test Analysis and Corrections for Unit 5 Lecture/notes/practice on Types of Bonds HMWK: Counting Atoms Applied Notes 18 Objective: Periodic Table trends Activities: Practice Sheet on Periodic Trends HMWK-Test Review Activity for Unit 5 25 Objective: To be able to distinguish among ionic, covalent and metallic bonds. Activities: Lecture/notes/practice on Types of Bonds Lewis Dot Structure Practice Wednesday 12 Objective: Investigate and describe general trends on the Periodic Table Activities: Lecture/notes Activity: Sorting Elements by Characteristics HMWK: Practice sheetdoing math w/Sci. Notation 19 Objective: Periodic Table trends Review for test Activities: Practice Sheet on Periodic Trends HMWK: Test Review Unit 5 26 Student Holiday Thursday 13 Same as Wednesday Friday 14 Objective: Investigate and describe general trends on the Periodic Table Activities: Graphing Atomic Radii, Ionization Energy and Electronegativity 20 Same as Wednesday 21 Objective: demonstrate proficiency in content of Unit 5 Activities: Test: Unit 5- Periodic Trends 27 28 Student Holiday December 1 Objective: Identify, compare, contrast ionic, metallic and covalent bonds Drawing Lewis Dot Structures and combining elements Activities: Applied Notes: Types of Bonds Practice drawing Lewis Dot Structures 8 Objective: VSEPR theory notes and Practice Activities: Molecular Geometry Practice 15 Objective: Final Review Activities: Final Exam Review 2 Objective: Identify, compare, contrast ionic, metallic and covalent bonds Drawing Lewis Dot Structures and combining elements Activities: Applied Notes: Types of Bonds Practice drawing Lewis Dot Structures 3 Objective: To draw Lewis structures to illustrate ionic bonds 9 Objective: VSEPR theory 10 Objective: Assessment Activities: Types of Bonds and Lewis Dot Structure Test Final Exam Review 11 Same as Wednesday 12 Objective: Final Review Activities: Final Exam Review Types of Bonds and Lewis Dot Structure review 16 17 18 19 Cycle 3 Final Exam Final Exam Final Exam Activities: Molecular Geometry Lab 4 Objective: Same as Wednesday Ionic bond formula combinations 5 Objective: To draw Lewis structures to illustrate covalent bonds Activities: Lewis Dot Structure and Ionic Practice Activities: Lewis Dot Structure and Ionic Practice Balancing Ionic Bonds Balancing Ionic Bonds Final Exam End of