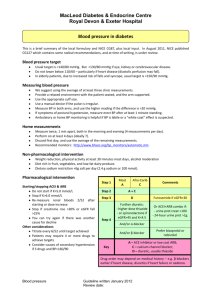
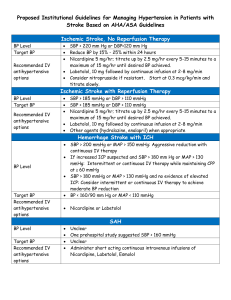
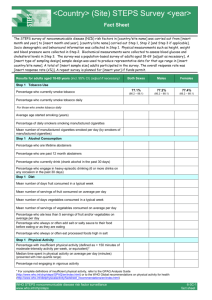
Sample Submission Form Hypertension in childhood and
advertisement

Sample Submission Form Hypertension in childhood and adolescence Physician Street Address: Postal Code: City: Country: Name: Specialty: Clinic / Practice: Phone: Fax: Email: Patient Information Parents‘ Contact Details Surname, Given Name(s): Name(s): Gender: male Street Address: Postal Code: City: Country: female Date of Birth (MM/DD/YYYY): . . Ethnicity (e.g. Italian, Moroccan, Japanese): Clinical Diagnosis 24-hour ambulatory blood pressure measurement: Diagnosis leading to Referral: Patient’s Age at Diagnosis: years months Blood pressure at Diagnosis: Date (MM/DD/YYYY): . . I: / mmHg II: / mmHg Earlier documented blood pressure measurements: Date (MM/DD/YYYY): . . / mmHg Date (MM/DD/YYYY): . / mmHg Current Symptoms: Phone: Email: 24-hour average blood pressure: / mmHg Average daytime blood pressure: / mmHg Average nighttime blood pressure: / mmHg Symptoms at Presentation: Other Diagnoses: . Current Medication: Medication Dose Differential Diagnoses: 1 Birth and Infant Development Gestational Age: weeks Perinatal History – Medical Events: At what age could the patient… …sit: months …crawl: months …walk freely: months …speak first words: months Umbilical Artery Catheter (UAC)? ja nein Apgar-Score (if known): Family History Are the parents consanguineous? yes no If yes, please indicate relationship (e.g. I° cousins). Are other family members affected by hypertension? yes no If so, who (Name, relationship, age, age of onset)? Number of living siblings: Miscarriages or siblings‘ early deaths? yes unknown If yes, please provide details (relationship, age). Is any of these diseases present in the family? Which family member(s) is/are affected? Would they be interested in taking part in the study? If so, how can we contact them? Stroke: Aneurysm: Early-onset myocardial infarction: Early-onset hypertension: Medical History and Physical Examination Date: . . unit Weight: unit Size: BMI: Blood Pressure Right arm: Left arm: % percentile % percentile / / mmHg mmHg Right leg: Left leg: / / mmHg mmHg 2 Other abnormalities: details: aortic coarctation developmental delay abdominal bruit sexual development disorder diabetes dysmorphic features (e.g. brachydactyly) dizziness/imbalance haematuria hypokalemia headache excessive consumption of liquorice nocturia left ventricular hypertrophy muscle weakness neurofibromatosis renal disease oedema thyroid disease sleep apnea sweating tachycardia transplantation (organ) other: none Laboratory Evaluation Haemogram value Erythrocytes unit reference range x 106/µl x 1012/l g/dl mmol/l % Haemoglobin Haematocrit Leukocytes Thrombocytes x 103/µl x 109/l /µl x 109/l Serum value unit Na+ mmol/l K+ mmol/l fasting glucose value eGFR mg/dl mmol/l mg/dl µmol/l ml/min BUN mg/dl creatinine reference range 3 mmol/l aldosterone ng/l renin ng/l plasma renin activity (PRA) ng/ml/h cortisol mg/dl catecholamines pg/ml cholesterol mg/dl mmol/l mg/dl mmol/l triglycerides Urine value unit reference range aldosterone µg/die protein µg/min mg/die µg/die catecholamines Further Examinations Cardiology Has an echocardiography been conducted? yes no If yes, please describe results. Renal biopsy? yes no If yes, please describe results. Nephrology Renal or renovascular imaging? yes no Dexamethasone suppression test? yes no If yes, please describe results. If yes, please describe results. Colour-coded duplex sonography of the kidney: Vmax: m/s RI right: RI left: Genetic Studies Adrenal imaging? yes no Karyotyping? yes, details: no Further genetic studies? If yes, please describe results. 4 Specimen Information Date obtained (MM/DD/YYYY): . . Sample material: Blood Saliva Number of tubes: Have other family members previously participated in our study? yes no If yes, please provide names. Please attach the following to your letter: This Sample Submission Form Specimen tube(s) with names and birth dates. For infants, 1-3 ml blood will be sufficient. Saliva kits are available upon request. Signed informed consent form Laboratory evaluation (haemogram, serum electrolytes, urinalysis, creatinine, urea, fasting glucose, renin, aldosterone, cortisol, catecholamines, triglycerides, cholesterol) Additional clinical information (e.g. letters, pedigree) Thank you very much! 5