Chemistry2 Midterm Study Guide: Jan. 18, 2013 Chemistry 2

Chemistry2 Midterm Study Guide: Jan. 18, 2013
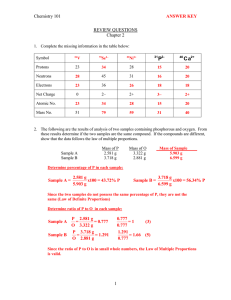
Chapter 1: MATTER & MEASUREMENT
1.1 – The Study of Chemistry
1.2 – Classifications of Matter
1.3 – Properties of Matter
1.4 – Units of Measurement
1.5 – Uncertainty in Measurement
1.6 – Dimensional Analysis
Chapter 3: STOICHIOMETRY
3.1 – Chemical Equations
3.2 – Patterns of Chemical Reactivity
3.3 – Atomic and Molecular Weights
3.4 – The Mole
3.5 – Empirical and Molecular Formulas
3.6 – Conversions using Balanced Chemical
Equations
3.7 – Limiting Reactants
Chapter 2: ATOMS, MOLECULES, & IONS
2.3 – Modern View of Atomic Structure
2.4 – The Periodic Table
2.5 – Molecules and Molecular
Compounds
2.6 – Ions and Ionic Compounds
2.7 – Naming Inorganic Compounds
Chapter 5: THERMOCHEMISTRY
5.1 – The Nature of Energy
5.2 – First Law of Thermodynamics
5.3 – Enthalpy
5.4 – Enthalpies of Reaction
5.5 – Calorimetry
5.6 – Hess’s Law
The Chem2 midterm is January 18, 2013 from 8:00-9:40 am (barring any weather-related schedule adjustments).
If you are currently enrolled in two science courses, you must notify the teacher which exam you will take during the Science block. If you have two science courses, the alternate times to take the test are: o January 17: 1:00-2:40 in Room 108 o January 18: 1:00-2:40 in Room 108 o January 22: Scheduled time between 8:00-2:00 in Library
The format of the midterm exam will be 65 multiple choice questions and 5 free response questions.
On the day of the exam, you will need a No. 2 pencil and a scientific (non-graphing) calculator. A periodic table will be provided, along with the formula sheet provided on the back of this paper
Chemistry 2 Midterm
2012
SI Prefixes
Name: _______________________________
Prefix
Giga-
Mega-
Kilo-
Deci-
Centi-
Milli-
Micro-
Nano-
Pico-
Femto-
Abbreviation
G
M k d c m
µ n p f
Meaning
10 9
10 6
10 3
10 -1
10 -2
10 -3
10 -6
10
10
10
-9
-12
-15
Example
1 gigameter (Gm) = 1 x 10 9
1 megameter (Mm) = 1 x 10 6
1 kilometer (km) = 1 x 10 3
1 decimeter (dm) = 0.1 m
m
1 centimeter (cm) = 0.01 m
m
m
1 millimeter (mm) = 0.001 m
1 micrometer (µm) = 1 x 10 -6 m
1 nanometer (nm) = 1 x 10 -9 m
1 picometer (pm) = 1 x 10 -12 m
1 femtometer (fm) = 1 x 10 -15 m
Convsersion Factors
K = °C + 273.15
°C =
𝟓
𝟗
(°F – 32)
°F =
𝟗
𝟓
(°C) + 32
Formulas
1 mL = 1 cm 3 1 Btu = 1.005 kJ
1 L = 1 dm 3 1 J = 1 kg·m 2 /s 2 𝒅𝒆𝒏𝒔𝒊𝒕𝒚 = 𝒎𝒂𝒔𝒔 𝒗𝒐𝒍𝒖𝒎𝒆 q = m·c·ΔT
ΔT = T f
– T i
% Yield = actual yield x 100
theoretical yield
Constants c
H2O
= 4.184 J/g·°C
Mole Conversions
Molar Mass: # g = 1 mol
ΔE = q + w
1 proton = 1.007 276 amu
1 neutron = 1.008 665 amu
4.184 J = 1 cal 1 amu = 1.6605 x 10 -27 kg 1 electron = 0.000 5486 amu
Avogadro’s Number: 6.02 x 10 23 particles = 1 mol
Molar volume: 22.4 L = 1 mol (GASES)
Molarity: # mol = 1 L (SOLUTIONS)
Molarity = moles solute
L solution
2