NOTES * MOLAR MASS AND MOLE CONVERSIONS

Name: ________________________________________
Chapter 10: MOLAR MASS AND PERCENT COMPOSTION
http://youtu.be/Qflq48Foh2w
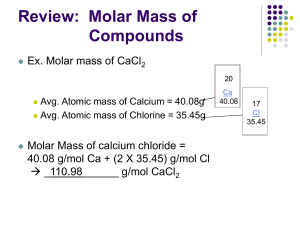
MOLAR MASS OF COMPOUNDS
What is the molar mass of a substance?
The molar mass of a substance is the mass (in grams) of 1 mole of that substance
(or, the mass of 6.02 X 10
23
particles of that substance)
REVIEW: What is the molar mass of the element Carbon?
What is the molar mass of Nitrogen gas?
To calculate MOLAR MASS of a compound…..
1.
Write the formula of the compound from the name
1.
Find atomic mass of each element
2.
MULTIPLY each atomic mass by the appropriate subscripts
3.
ADD (units are g/mole) (the amount of grams in one mole)
EXAMPLES:
Find molar mass of water.
2 mol H
1 mol O
2(1.01g/mol) =
1(16.00g/mol) =
FORMULA H
2.02g/mol
16.00g/mol
18.02g/mol
2
O
So, the Molar Mass of H
You Try: Find molar mass of sulfuric acid
2
O = 18.02 g/mol
2 mol H
1 mol S
4 mol O
FORMULA H
2
For ionic compounds, reminder to criss cross in order to find the chemical formula.
SO
4
1
Use parentheses for poly’s
Record your masses to the hundredth’s place!!!!
Do 1 st
1.
sodium hydroxide 1 st write the formula by crisscross
2 nd
add up the molar mass very neatly!
FORMULA: __
NaOH
SCRAPWORK
_____ Na
+1
(OH)
-1
Na: 1 ( 22.98) = 22.99 NaOH
O: 1 ( 16.00) = 16.00
40.0 g/mol
H: 1 ( 1.01) = 1.01
2.
magnesium hydroxide 1 st
write the formula by crisscross
2 nd
add up the molar mass very neatly!
FORMULA: __ Mg(OH)
2
______ Mg
+2
(OH)
-1
Mg: 1 ( 24.31) = 24.31 Mg(OH)
2
O: 2 ( 16.00) = 32.00
58.47 g/mol
H: 2 ( 1.01) = 2.02
3.
calcium cyanide 1 st
write the formula by crisscross
2 nd
add up the molar mass very neatly!
FORMULA: _____________
4.
magnesium phosphate 1 st
write the formula by crisscross
2 nd
add up the molar mass very neatly!
FORMULA: _____________
5.
iron(III) dichromate 1 st write the formula by crisscross
2 nd add up the molar mass very neatly!
FORMULA: _____________
6.
ammonium hydroxide 1 st write the formula by crisscross
2 nd
add up the molar mass very neatly!
FORMULA: _____________
7.
dinitrogen pentoxide covalent so don’t criss cross
2 nd
add up the molar mass very neatly!
FORMULA: _____________
2
PER CENT COMPOSITION OF AN ELEMENT IN A COMPOUND
http://youtu.be/lywmGCfIUIA
Find percent composition of C
2
H
6
O
To calculate PERCENT COMPOSITION…….
1.
Write the formula of the compound
2.
Find the molar mass of the each element
3.
Find the TOTAL MOLAR MASS of the compound
4.
DIVIDE the molar mass of each element by
TOTAL MOLAR MASS of the compound.
5.
MULTIPLY each by 100 to find percent of each
EXAMPLES:
FORMULA: ____ Ca(CN)
2
______
1 ST Find molar mass NEATLY
Ca – 1 x 40.08g = 40.08g 2 ND percent
(PART/WHOLE)(100) = %
C
2
– 2 x 12.01 g = 24.02g
(40.08/92.12) x 100 = 43.51%
N
2
– 2 x 14.01g = 28.02g
% C = _ (24.02/92.12) x 100 = 26.07%
% N = _ (28.02/92.12) x 100 = 30.41%
92.12 g/mole
From per cent composition, we can determine the mass of an element in a given sample of that compound:
How many grams of calcium are in 40.0g of calcium cyanide?
Formula to use = [(percent (in decimal) of Ca in Compound)(40.0 g of the compound)]
% Ca = 43.51%
in decimal it is 0.4351
[(0.4351)(40.0g) = 17.4g of Ca a.
Find the per cent composition of magnesium phosphate:
FORMULA: ______________________ % Mg = __________
% P = __________
% O = __________
How many grams of magnesium are in 350g of magnesium phosphate?
3
***** Extension: From percent composition, we can determine the mass of a given sample of that compound if we know the mass of an element in that sample:
What mass of magnesium phosphate contains 15g of magnesium?
YOU TRY SOME:
1. Find the molar mass of each compound (review): a.
lithium carbonate b.
calcium nitrate c.
tin (IV) sulfate
2. Find the percent composition of tin (IV) sulfate .
____________________
_____________________
______________________
% Sn = __________
% S = __________
% O = __________
How many grams of tin are in 250g of tin (IV) sulfate?
4
MOLE CONVERSIONS Moles/Mass, Moles/Particles, Moles/Volume
1. mole
grams
http://youtu.be/CMnkSb2YsXI
2. mole
grams
http://youtu.be/0RXB8xNmJNM
3. mole
particles
http://youtu.be/HMAOrGpkTsQ
4. mole
particles
http://youtu.be/kGNtnq0kGKk
5. mole
liters
http://youtu.be/Y8e7T09SKZ0
Mass Mole
1 mole O
2
32.00g O
2
1 mole CO
2
44.01g CO
2
4.00g He
1 mole He
180.00g C
6
H
12
O
6
1 mole C
6
H
12
O
6
Volume Mole
(at STP)
1 mole O
2
22.4 liters O
2
1 mole CO
2
22.4 liters CO
2
22.4 liters O
2
1 mole O
2
22.4 liters CO
2
1 mole CO
2
Particles Mole
1 mole O
2
6.02 x 10 23 molecules O
2
1 mole He
6.02 x 10 23 atoms of He
6.02 x 10 23 formula units NaCl
1 mole NaCl
6.02 x 10 23 Br ions
1 mole Br ions
5
MOLE/MASS and MASS/MOLE/PARTICLE CONVERSIONS USING MOLAR MASS
STEP 1: Write the correct formula
STEP 2 : Determine the molar mass
STEP 3 : Use dimensional analysis to convert
Conversion Factors
1 mole = 6.02 x 10
23
molecules
1 mole = 6.02 x 10 23 formula units
1 mole = 6.02 x 10
23
atoms
1 mole = molar mass in grams
EXAMPLES:
1.
Find the mass of 4.50moles of diphosphorus pentoxide.
2 nd – Set up the bridge to convert moles into mass (grams)
1 st – Write the formula.
If it is covalent , then don’t criss cross. If it is ionic, then criss cross. Then add up molar mass
P
2
O
5
2(30.97) + 5(16.00)= 141.94 g. (this goes near the gram in the
4.50 mol P
2
O
5
x 141.94 g mol P
2
O
5
= 639 g P
2
O
5
1 mol P
2
O
5 conversion factor.
2.
How many moles is 250.0g of copper (II) sulfate?
2 nd – Set up the bridge to convert mgrams into moles
250.0 g CuSO
4
x 1 mol CuSO
4
= 1.57 mol CuSO
159.57 g CuSO
4
4
___639 g P
2
O
5
_
1 st – Write the formula. If it is covalent , then don’t criss cross. If it is ionic, then criss cross. Then add up molar mass
Cu +2 (SO4) -2 (charges wipe out)
CuSO
4
1(63.5) + 1(32.07) + 4(16.00)= 159.57 g. (this goes near the gram in the conversion factor.
3.
Find the mass of 0.545moles of calcium cyanide. ___1.57 mol CuSO
4
___
4. How many molecules are in 110g of diphosphorus pentoxide? _______________
(Hint: go through the mole)
YOU TRY SOME:
1.
575g of sodium sulfate to moles ________________
2.
0.025moles of phosphorus pentachloride to grams ________________
6
3.
15.0g of iron(III) nitrate to moles
4.
8.02 x 10
23
molecules of carbon disulfide to grams
MOLES AND VOLUME OF MATTER
Avogadro’s Hypothesis:
STP:
________________
________________
How many is a mole?
How heavy is a mole?
1 mole = 6.02 X 10 23 atoms, molecules, formula units, or particles
1 mole = molar mass in grams
How much space does a mole occupy? 1 mole = 22.4 L (for a gas at STP)
YOU TRY SOME-Make the following mole conversions: a.
14.0L of nitrogen gas at STP to moles b.
2.5g of chlorine gas at STP to molecules c.
2.24 x 10
25 atoms of neon at STP to liters d.
13.3L of fluorine gas at STP to grams
7
EMPIRICAL AND MOLECULAR FORMULAS -
http://youtu.be/wnRaBWvhYKY?list=PL3hPm0ZdYhyyWLwHs-N8En7iTuJ9jZQqr http://youtu.be/ucU5PD6W3Ik?list=PL3hPm0ZdYhyyWLwHs-N8En7iTuJ9jZQqr http://youtu.be/J_MtVs0aBdU?list=PL3hPm0ZdYhyyWLwHs-N8En7iTuJ9jZQqr
Compare the Empirical Formula and Molecular Formula of each compound above.
What is an Empirical Formula?_______________________________________________________________
What is a Molecular Formula?_______________________________________________________________
YOU TRY SOME: Given the following molecular formulas , determine the empirical formula of each compound. a. P
4
O
10 b. C
6
H
12
O
6 c. C
3
H
6
O
__________ _________ ________
http://youtu.be/lyWAGMEKzSY
If we know the empirical formula and the molar mass of a compound, we can determine the molecular formula of that compound by finding the ratio of the given molecular molar mass to the molar mass of the empirical formula.
For example: Given the empirical formula CH and the molar mass 78g/mol, find the molecular formula.
STEP 1: Find molar mass of the empirical formula: 12 g/mol + 1 g/mol = 13 g/mol
STEP 2: Divide the given molecular molar mass by the empirical molar mass (from step 1): 78 g/mol = 6
13 g/mol
STEP 3: Round your answer in Step 2 to the nearest whole number (this is the ratio): 6
STEP 4: Multiply all subscripts in the empirical formula by the ratio from step 3: C
6
H
6 http://youtu.be/IYDTbqg5IG4
8
These are easier than the video since the empirical formula is given in these next few problems:
Determine the molecular formula of each compound
1.
The empirical formula of a compound is CH
2
O. Its molar mass is 360g/mol. Find its molecular formula.
2.
The empirical formula of a compound is P
2
O
5
. Its molar mass is 284g/mol. Find its molecular formula.
3. The empirical formula of a compound is CH
2
O. Its molar mass is 180g/mol. Find its molecular formula.
4. The empirical formula of a compound is HO . Its molar mass is 34g/mol. Find its molecular formula.
HW#1: REVIEW- NAMING AND MOLE CONVERSIONS
9
NAME
Potassium permanganate
Ionic or Covalent? FORMULA
Tricarbon octahydride
Iron (III) carbonate
Tetraphosphorus decoxide
Aluminum sulfate
Copper (II) sulfite
Magnesium iodide
Tetraphosphorus decoxide
Mole conversions: (you may want to go back and look at your previous notes on mole conversions) a. Find the mass of 1.25 moles of K (mole → g) b. How many moles is 2.50g of oxygen? (hint: oxygen is diatomic ) (g → mole) c. Find the number of atoms in 5.45 g of phosphorus. (g → mole → atoms)
HW #2 MOLAR MASS/ PER CENT COMPOSITION
10
1. Label each compound below as ionic or molecular. Write the formula and determine the molar mass of each compound.
COMPOUND IONIC/
MOLECULAR?
FORMULA MOLAR MASS barium sulfate tricarbon octahydride iron (III) carbonate tetraphosphorus decoxide strontium phosphate
2. Find the per cent composition of iron (III) carbonate.
% Fe =
% C =
% O =
How many grams of iron are in 125g of iron (III) carbonate?
What mass of iron(III) carbonate contains 10.0g of iron?
HW #3 PERCENT COMPOSITION
11
Determine the percent composition of each of the compounds below:
1.
KMnO
4
% K = ______________
% Mn = _____________
% O = ______________
2.
HCl
% H = ______________
% Cl = ______________
3.
Mg(NO
3
)
2
% Mg = _____________
% N = ______________
% O = ______________
4.
(NH
4
)
3
PO
4
% N = _____________
% H = ______________
% P = ______________
% O = ______________
5.
Al
2
(SO
4
)
3
% Al = ______________
% S = ______________
% O = ______________
Solve the following problems:
6.
How many grams of oxygen can be produced from the decomposition of 100g of KClO
3
?
7.
How much iron can be recovered from 25.0g of Fe
2
O
3
?
8.
How much silver can be produced from 125g of Ag
2
S?
HW #4 CONVERSION PRACTICE
12
1. Write the formula for each compound below. Then determine its molar mass.
FORMULA MOLAR MASS a. calcium sulfate b. aluminum cyanide c. phosphorus triiodide
2. Use the molar masses you found in Part 1 to make the following conversions: a. Find the mass of 1.25moles of calcium sulfate. b. How many moles is 2.50g of aluminum cyanide? c. Find the mass of 0.750moles of phosphorus triiodide. d. How many molecules is 12.50g of phosphorus triiodide e. Find the number of formula units in 10.0g of calcium sulfate.
13
HW #5 MIXED MOLE CONVERSION PRACTICE
1.
Convert the quantities below to moles: a.
14.0g of lead
b. 20.5g of lithium hydroxide
c. 14.0L of oxygen gas at STP
d. 15.0g of calcium nitrate
2. Find the mass of: a. 10.0L of hydrogen gas at STP b. 4.5 moles of sodium sulfate c. 3.21 x 10
22
molecules of carbon tetrachloride
_____________________
______________________
_______________________
_______________________
_______________________
_______________________
_______________________
14
PER CENT SUGAR IN BUBBLE GUM NAME:
DATE:
PERIOD:
BACKGROUND: In this lab you are going to design and carry out an experiment to determine the percentage of sugar in bubble gum. To do so, you will assume that all of the flavoring removed from bubble gum during chewing is sugar. The chemical formula for sucrose, the sugar in bubble gum, is C
12
H
22
O
11
. After determining the percentage of sugar in bubble gum, you will then determine the mass of carbon and the number of carbon atoms in the sugar in a piece of bubble gum.
PROCEDURE: Write out the procedure (in words) you will follow in the space below. Include all details.
1.__________________________________________________________________________________
2.__________________________________________________________________________________
3. .__________________________________________________________________________________
4. .__________________________________________________________________________________
5.
DATA: Set up a data table in the space below to record all necessary data.
15
CALCULATIONS: Show and explain all work. Calculations must be neat and easy to follow.
a. mass of sugar in your piece of gum: (show calculation!) a.
% sugar in bubble gum [% = (part/whole) x 100) ] (show calculation based on your data!) b.
% carbon in sugar (C
12
H
22
O
11
) (show calculation – use your class notes on % composition) c.
mass of carbon in the sugar in your piece of bubble gum (show calculation, use class notes)
(HINT: What per cent of sugar is carbon? How many grams of sugar were in your gum? ) d.
Find the # of atoms of carbon from the grams you calculated in “d”. (use dimensional analysis)
CONCLUSIONS:
1.
What did you learn from this experiment?
2.
List two sources of error and describe the effect each had on your results. (Would the error make the per cent sugar reported too high or too low and why). a. b.
16
HYDRATES
A hydrate has H
2
0 molecules attached to the solid form. If we heat a hydrate, we can drive off the water
(and be left with the dry anhydrous form).
The following information is obtained in a CuSO
4
• X H
2
O hydrate lab:
22.00g: mass of the crucible and cover
24.50g: mass of crucible, cover , and hydrate
23.59g: mass of crucible, cover, and anhydrous powder
1) Find the mass of hydrate
2) Find the mass of anhydrous powder.
3) Find the mass of the water driven off.
4) Find the empirical formula of the hydrate.
17