disclosure of financial relationships with commercial interest
advertisement
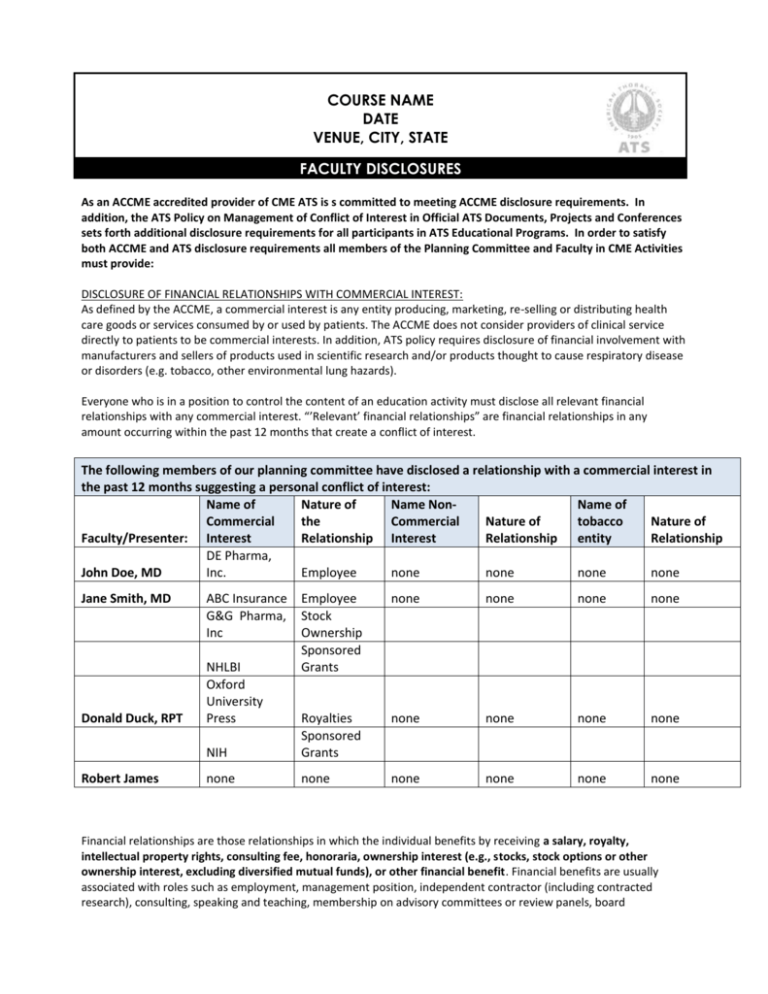
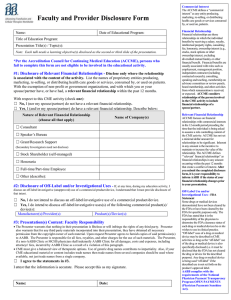
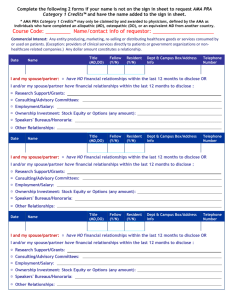
COURSE NAME DATE VENUE, CITY, STATE FACULTY DISCLOSURES As an ACCME accredited provider of CME ATS is s committed to meeting ACCME disclosure requirements. In addition, the ATS Policy on Management of Conflict of Interest in Official ATS Documents, Projects and Conferences sets forth additional disclosure requirements for all participants in ATS Educational Programs. In order to satisfy both ACCME and ATS disclosure requirements all members of the Planning Committee and Faculty in CME Activities must provide: DISCLOSURE OF FINANCIAL RELATIONSHIPS WITH COMMERCIAL INTEREST: As defined by the ACCME, a commercial interest is any entity producing, marketing, re-selling or distributing health care goods or services consumed by or used by patients. The ACCME does not consider providers of clinical service directly to patients to be commercial interests. In addition, ATS policy requires disclosure of financial involvement with manufacturers and sellers of products used in scientific research and/or products thought to cause respiratory disease or disorders (e.g. tobacco, other environmental lung hazards). Everyone who is in a position to control the content of an education activity must disclose all relevant financial relationships with any commercial interest. “’Relevant’ financial relationships” are financial relationships in any amount occurring within the past 12 months that create a conflict of interest. The following members of our planning committee have disclosed a relationship with a commercial interest in the past 12 months suggesting a personal conflict of interest: Name of Nature of Name NonName of Commercial the Commercial Nature of tobacco Nature of Faculty/Presenter: Interest Relationship Interest Relationship entity Relationship DE Pharma, John Doe, MD Inc. Employee none none none none Jane Smith, MD Donald Duck, RPT Robert James ABC Insurance G&G Pharma, Inc Employee Stock Ownership Sponsored Grants none none none none none none none none NIH Royalties Sponsored Grants none none none none none none NHLBI Oxford University Press Financial relationships are those relationships in which the individual benefits by receiving a salary, royalty, intellectual property rights, consulting fee, honoraria, ownership interest (e.g., stocks, stock options or other ownership interest, excluding diversified mutual funds), or other financial benefit. Financial benefits are usually associated with roles such as employment, management position, independent contractor (including contracted research), consulting, speaking and teaching, membership on advisory committees or review panels, board membership, and other activities from which remuneration is received, or expected. ACCME considers relationships of the person involved in the CME activity to include financial relationships of a spouse or partner. (ACCME added March 2005). Anyone who does not disclose all relevant financial relationships is disqualified from being a planning committee member or a teacher in a CME Activity. All conflicts of interests must be resolved prior to the activity. DISCLOSURE OF NON-COMMERCIAL, NON-GOVERNMENTAL INTERESTS Everyone who is in a position to control the content of an education activity must disclose if they have received support within the past three years from a non-commercial (non-profit) source that has an interest in the scope of the official activities of the ATS (e.g., foundation or other nonprofit source). TOBACCO INDUSTRY RELATIONSHIPS ATS does not accept as a planner or oral presenter for its conferences anyone who has a current relationship with a tobacco entity, or has had one within the preceding 12 months. All Planners and Faculty must disclosure of all financial and non-financial affiliations with tobacco entities during at least the past three years. OFF-LABEL USE OF DRUGS (Faculty Presenters Only) Faculty presenters are required to disclosure of whether their presentation will include discussion of off-label use of drugs or investigational use of a drug or device not approved by the FDA. Disclosure of Discussion of “off-label” use of drug: Faculty/Presenter: Generic Product Name Off-label Use(s) The following disclosures of financial relationships had been provided by course faculty at the time this syllabus went to press. Faculty members have been informed that, in the event that their disclosures were not received in time for inclusion, it is his or her responsibility to announce disclosures at the start of their presentation.







![June 2013 [DOCX 24.38KB]](http://s3.studylib.net/store/data/006990913_1-45414924984da7777020f5c1725fdda9-300x300.png)

