EDTA Titration of the Hardness of Water
advertisement

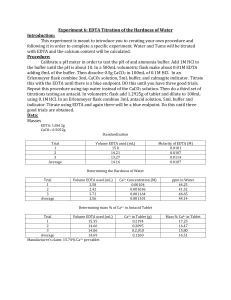
Quantitative Analytical Chemistry Lab Six EDTA Titration of the Hardness of Water Purpose: We will be making our own procedure to help determine the hardness of water for this lab. The data will be done in complete form and the procedure will be written in a form which will allow for a colleague to repeat it. The percent Ca2+ will be found and will be used as the determinant of the hardness of water. Procedure: 1) Calibrate the pH by pouring in about 50mL of 6M ammonia as the buffer. 2) Using the solutions of 4.01, 7, and 10.01 calibrate the ammonia until enough HCl was added to get a pH of 10. 3) Weigh out 0.5 grams of Calcium Carbonate (CaCO3). 4) Add 5mL of ammonia buffer to the CaCO3. 5) You need 0.01 M of EDTA, calculate the mass using a 250mL dilution. 6) You need 0.1M of HCl to be added to the CaCO3 to form CaCl2. 7) Take the EDTA solution and add 5mL of ammonia buffer. 8) 3 trials will be done using the CaCl2 (3mL), ammonia (5mL), and a few drops of calmagite indicator and titrated with EDTA. 9) The color will turn pink/dark violet from the calmagite indicator addition and as the EDTA is added, it will turn blue. 10) Take 25mL of tap water and add ammonia buffer (3mL). 11) Take 3 separate 250mL Erlenmeyer flasks and add 5mL of the solution of tap water and ammonia and a few drops of calmagite indicator to each. 12) Titrate each of the flasks with EDTA 9it will turn pink/dark violet to blue). 13) Mass out a TUMS tablet. 14) Crush the TUMS tablet and add it to a 100mL volumetric flask. 15) Add 5mL of ammonia buffer to the 100mL volumetric flask and then dilute to the mark. 16) 5mL of each solution will be peppered into a 250mL Erlenmeyer flask. 17) Then add another 1mL of ammonia buffer to each of the 250mL flasks and add a few drops of calmagite indicator to each flask. 18) Titrate these with EDTA (it will turn from pink/dark violet to blue). Data: Mass of EDTA: 0.9360 g Mass of CaCO3: 0.5007 g Mass of Antacid: 3.1412 g Standardization of EDTA Trial Number 1 2 3 Average Amount EDTA Added (mL) 2.00 2.02 1.98 2.00 Molarity of EDTA (M) 0.07504 0.07432 0.07583 0.07504 Tap Water Titration Trial Number 1 2 3 4 Average (best three) Amount EDTA Added (mL) 2.00 2.11 1.51 2.07 2.06 Ca2+ Molarity (M) ppm 0.0060032 0.0063334 0.0045324 0.0062133 0.0061833 240.60 253.83 181.65 249.02 247.81 Titration of TUMS Trial 1 2 3 Average Amount EDTA Added (mL) 2.00 1.69 2.04 1.91 % Ca2+ in sample 3.83% 3.24% 3.91% 3.66% Calculation: Mass of EDTA needed for a 0.1M solution: Equation: Molar Mass of EDTA x 0.01 mol EDTA x 0.250 L = mass of EDTA 1 mol of EDTA x 1 L Example: 374.74 g EDTA x 0.01 mol EDTA x 0.250 L = 0.9356 g EDTA 1 mol of EDTA x 1 L Grams of Calcium Carbonate needed for CaCl2 solution: Equation: Molarity of CaCL2 x mL of CaCL2 used x mol CaCO3 x Molar mass of CaCO3 mols of CaCl2 x mol of CaCO3 = mass of CaCO3 Example: 0.1 M CaCL2 x 0.1mL of CaCL2 used x 1 mol CaCO3 x 100.09 g CaCO3 2 mols of CaCl2 x 1 mol of CaCO3 =0.5 g CaCO3 Molarity of EDTA: Equation: Example: g CaCO3 x 1 mol CaCO3 x 1 mol solution x L CaCL2 100.09 g CaCO3 x 0.1L solution x L EDTA added = M EDTA 0.5007 g CaCO3 x 1 mol CaCO3 x 1 mol solution x 0.003 L CaCL2 100.09 g CaCO3 x 0.1L solution x 0.002L EDTA added = 0.07504 M EDTA Ca2+ Concentration in Tap Water: Equation: Example: L EDTA added x M EDTA x 1 mol Ca2+ x 1 1 mol EDTA x 0.025 L = Ca2+ Molarity 0.002 L EDTA added x 0.07504 M EDTA x 1 mol Ca2+ 1 mol EDTA x 0.025 L = 0.0060032 M Ca2+ Hardness of Water: Equation: Example: Ca2+ Molarity x grams Ca2+ x 1000 mg 1 mol Ca2+ x 1 g = ppm (hardness of water) 0.0061833 Ca2+ Molarity x 40.078 grams Ca2+ x 1000 mg 1 mol Ca2+ x 1 g = 247.81 ppm Claimed amount of Ca2+ in tablet: Equation: Example: Equation: Example: g CaCO3 x 1 mol CaCO3 x 1 mol Ca2+ x g Ca2+ Molar Mass CaCO3 x 1 mol CaCO3 x 1 mol Ca2+ = Ca2+ in the tablet 0.5007 g CaCO3 x 1 mol CaCO3 x 1 mol Ca2+ x 40.078 g Ca2+ 100.09 g CaCO3 x 1 mol CaCO3 x 1 mol Ca2+ g Ca2+ mass of tablet 0.2005 g Ca2+ 3.1412 g x 100% = Amount of Ca2+ in the tablet x 100% = 6.38% Ca2+ in the tablet = 0.2005 g Ca2+ Amount of Ca2+ in the sample: 20 parts/solution Equation: Example: L EDTA added x M EDTA x 1 mol Ca2+ x Molar Mass Ca2+ x 20 parts 1 mol EDTA x 1 mol Ca2+ = grams Ca2+ 0.00191L EDTA added x 0.07504 M EDTA x 1 mol Ca2+ x 40.078 g Ca2+ x 20 parts 1 mol EDTA x 1 mol Ca2+ = 0.1149 g Ca2+ Equation: Example: g Ca2+ Mass of tablet 0.1149 g Ca2+ 3.1412 g x 100% = amount of Ca2+ in sample x 100% = 3.66% Ca2+ in the sample Conclusion: The purpose of this lab was to find the hardness of tap water. Hard water is water that contains many cations. In this lab, Ca2+ was measured to determine the water’s hardness. The ppm of Ca2+ in tap water that I found was 247.81 ppm. This amount shows that the cations in our lab’s tap water are relatively high. This amount of ppm is off, however, and I explain why in my next paragraph. I believe the ppm should actually be even higher. Another reason, besides the main one I will explain later, the ppm amount was high (even though there was an error that if corrected would have made the ppm even higher) is because we took samples of water from the faucet without letting the water run first. There is a buildup of cations in the faucet water and this went into our sample. The Tums tablet’s claimed the amount of Ca2+ in the tablet was found to be 6.38%. From this experiment it was determined that the actual amount of Calcium in the tablet was an average of 3.66%. These numbers are very small and are due to errors within my experiment. First, I miscalculated the amount of grams of CaCO3 needed for the CaCl2 solution. I calculated the grams of CaCO3 with a 1:2 ratio for CaCO3 to CaCl2 when it should have been calculated with a 1:1 ratio. Because of this miscalculation, my Molarity of EDTA was off, which caused my Ca2+ concentration of tap water to be off, which caused my hardness of water (ppm) to be off, which caused my amount of Ca2+ in the tablet and sample to be off. This miscalculation is what also could have lead to our low mL of EDTA needed for our titration (because the color did change in the titration, just with only a tiny bit of EDTA needed).