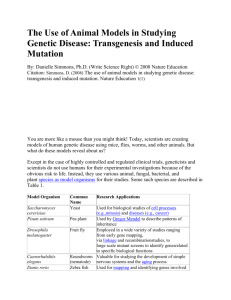
101101_Cover_letter
advertisement

LIQUN LUO Professor of Biology Investigator, Howard Hughes Medical Institute October 12, 2010 Editorial Office Nature Genetics Dear Nature Genetics Editors, We are submitting a manuscript entitled “Site-specific integrase-mediated transgenesis in mice via pronuclear injection” to be considered for publication as a Technical Report in Nature Genetics. This manuscript describes a new method that we believe will change the way people perform transgenesis in mice. The standard procedure for producing transgenic mice, by injecting DNA into zygotic pronuclei, has served the mouse community for about 30 years. Although powerful, this method has important limitations because it cannot control the insertion site, integrity and copy number of transgenes. Our method solves all these problems by using the C31 integrase to mediate transgenesis. We first produced mouse strains with the attP sites knocked-in at two different genomic loci, and then injected a recombinant DNA containing an attB site into embryos from these strains. We show that single-copy intact transgenes integrate at these predetermined loci with high frequency (equivalent to or better than the frequency of standard transgenesis), that these transgenes are faithfully transmitted across generations, and that a marker gene driven by a ubiquitous promoter enables global high-level transgene expression. Conceptually, our method is similar to the one described in Drosophila a few years ago. The C31-mediated transgenesis in flies is becoming the preferred method compared to classic Pelement mediated transgenesis that has served the Drosophila community since the early 1980s. Because the P-element method has much better control of transgene expression than the standard transgenesis procedure in mice, we expect that our method will be an even larger step forward in mouse transgenesis compared to the integrase-based transgenesis in flies. Moreover, turning this concept into reality in mice was not trivial. We identified loci that support global expression. We produced mice that are homozygous for the alleles targeted with attP sites. We also spent a large amount of time refining the procedure to remove expression variability and silencing. (We obtained our first integrase-mediated transgenic mice more than two years ago, but have been continually refining the method since then). We believe that this method is finally ready for use by the community. In anticipation of a potential large amount of requests by the community once our paper is published, we are already in the process of setting up procedures with companies to efficiently distribute the mice we have produced for integrase-mediated transgenesis. Department of Biology 385 Serra Mall Stanford, CA 94305-5020 T 1.650.723.6645 F 1.650.724.4213 lluo@stanford.edu In addition to the new method for transgenesis, we also describe the profound silencing and expression variability of our transgene in the proximity of a tissue-specific promoter and plasmid’s bacterial backbone. To our knowledge this is the first systematic and quantitative assessment of such effects in a chromosomally-integrated transgene in mammals in vivo. We show how to circumvent these effects, which have important implications for mammalian transgenesis including gene therapy in humans. Thus, the biological findings, which were obtained during the development of our new technology, add important value to this study. We believe that our study will be greatly appreciated by the mouse genetics community, as well as by researchers interested in regulation of gene expression in general. We recommend the following scientists as potential reviewers: Mario Capecchi (co-inventor of the mouse knockout procedure); Phillipe Soriano (discovered the Rosa26 locus); Allan Spradling (co-inventor of the P-element-mediated transgenesis in Drosophila); Hugo Bellen (co-inventor of integrase-mediated transgenesis in Drosophila). Katheryn Anderson (pioneer of forward genetic screens in Drosophila and mice). Sincerely yours, Liqun Luo, PhD



![Historical_politcal_background_(intro)[1]](http://s2.studylib.net/store/data/005222460_1-479b8dcb7799e13bea2e28f4fa4bf82a-300x300.png)