Chem 3210-01 Quantitative Analysis Spring, 1997

CHEM 3210
Time: TR,
QUANTITATIVE ANALYSIS
9:25-10:40 AM
Instructor: Dr. Mehnaaz F. Ali
NCF Annex, Rm 175
SPRING, 2014
NCF Room 315 Tel: 520-7378
Office hours: M 11:00 – 12:00, W 10:00 - 12:00, Th 11:00 - 12:00 or by appointment
Course Description: Theory and techniques of chemical analysis including evaluation of data, titrimetric methods, electrochemical analysis, spectrometry, and chromatography.
Prerequisite: Chem 1010/1010DR/1011LB, Chem 1020/1020DR/1020LB.
Required Text: Quantitative Chemical Analysis by Daniel C. Harris, Freeman
Course Objective: To present the theory and principles pertaining to the analytical process by providing a foundation for gathering and interpreting reliable experimental data. Students will learn the theory and practice of classical as well as modern analytical techniques that are commonly used in quantitative analysis.
Course Requirements: Students are required to attend all lectures and be present for all quizzes.
Students are required to take three hour-exams and a comprehensive final exam. There will be no makeup exams for any reason.
Course Evaluation: The final course grade will be based on the total points earned.
Three hour exams (3x 100 pts)
Unannounced quizzes (count 5 from 6-7 total)
Comprehensive final exam
300
50
100
If an exam is missed for an excused absence, the final exam will count as 200 pts.
90-100 % A
80-89 %
70-79 %
60-69 %
< 60%
B
C
D
F
Policy for Cheating: If a student's examination paper gives evidence of not being completely his/her own work, he/she may be given an F for the course. A student who communicates with anyone during an examination or test, unless with the permission of the instructor, may be immediately dismissed from the room and given an F. Attempts to read from other's paper, bringing study materials into the examination room without the instructor's permission will also result in an F. If a student is found to have brought study materials into the examination room without the instructor’s permission, it may be assumed that he/she intended to use such materials unlawfully, and he/she may be penalized accordingly.
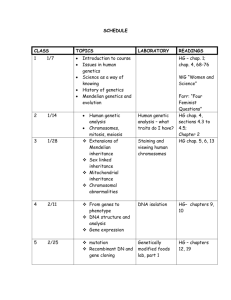
Tentative Lecture Schedule:
1/14
Tuesday
Introduction and Chap 0 and 1
1/21
Chap 4: Statistics
1/28 Chap 4: Statistics
Chap 5: Quality Assurance
2/4
Chap 6: Chemical Equilibrium
Thursday
1/16
Chap 3: Experimental Error
1/23
Chap 4: Statistics
1/30 Chap 1: Titrations and
Stoichiometric calculations (Review)
2/11
EXAM 1
2/18
Chap 9: Polyprotic Acid-Base Equil
2/25
Chap 10: Acid-Base Titrations
3/4
Mardi Gras Holidays
3/11
Chap 11: EDTA Titrations
3/18 Chap 13: Fundamentals of EChem
2/6 Chap 7: Activity and System Equil
Chap 8: Monoprotic Acid-Base Equil
2/13
Chap 8: Monoprotic Acid-Base Equil
2/20
Chap 10: Acid-Base Titrations
2/27
Chap 11: EDTA Titrations
3/6
Mardi Gras Holidays
3/13 EXAM 2
3/25 Chap 14: Electrodes and
Potentiometry
4/1
Chap 15: Redox Titrations
4/8
4/15
Chap 18: Spectrophotometry
Chap 22: Separations
3/20 Chap 14: Electrodes and
Potentiometry
3/27
Chap 15: Redox Titrations
4/3
Chap 17 : Spectrophotometry
4/10
EXAM 3
4/17
SPRING BREAK
4/22
Chromatography
4/24
Precipitation Titrations
Final Exam: 8:00-10:00 AM, Wednesday, April 30, 2014