attachment h
advertisement

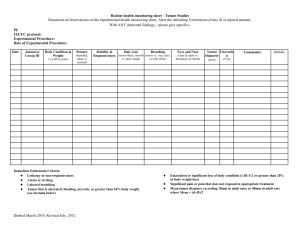
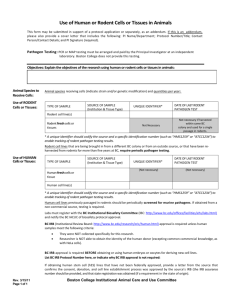
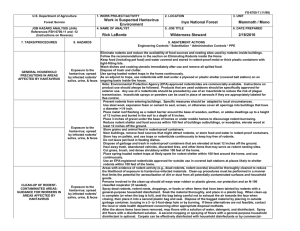
2015 ATTACHMENT H TUMORS, STEM CELLS and OTHER BIOLOGICAL MATERIALS Contamination of biological specimens such as tumor cell lines, stem cells, and sera with rodent pathogens can cause devastating disease outbreaks when laboratory animals are implanted with these materials. Biological materials derived from or passaged through rodents must be tested by Rat or Mouse Antibody Production or PCR to detect rodent infectious agents before being implanted into rodents. (Tissue culture Mycoplasma testing is not adequate). An exception may be made for biological materials originating from animals housed in the same animal facility. As an alternative, animals may be implanted with untested materials if they are housed in a dedicated quarantine facility for the duration of the experiment. However, limited quarantine space may preclude this option. Note: Answer sections will expand. 1. Are non-cellular biological materials being administered to rodents? YES NO If yes, what is the species of origin and source of the biological material? 2. Are rodents being injected with cells? YES NO If yes, are these cells derived from rodents? If the cell line was derived from humans or other animals, has it been passaged through rodents? YES NO What are the cells and where did you obtain them? What is the injection site in the animals? 3. Has this biological material been tested for rodent pathogens? YES NO If no, contact one of the university veterinarians regarding availability of quarantine space. If yes, please attach a copy of the testing results. All testing results must be attached, or if not available, must be forwarded to facility managers prior to use in animals. I certify that the tested materials to be used have not been passed through rodent species since testing. To the best of my knowledge the material remains uncontaminated with rodent pathogens. YES NO 2015 Tumor Endpoints If tumours are part of this protocol, please use the following endpoint information to assist in completing Section 7 of the protocol form. Humane endpoints must be established and described under Section 7 of the animal protocol. The information below is provided to assist you in completing Section 7. For tumor studies, animals should be euthanized before their tumor burden becomes excessive and before the animals become debilitated. The appearance of any of the following clinical signs should trigger the decision to euthanize tumor-bearing animals unless strong scientific justification for later endpoints is provided: • • • • • • • • Tumor size > 10% of body weight (about 1.5 cm in a 25g mouse) Weight loss of more than 15% of body weight (corrected for the weight of tumor) Abdominal distension approximately equal to that of a near-term female Ulceration or necrosis of the tumor Impaired mobility due to size or location of the tumor Respiratory distress Blood stained or mucopurulent discharge from any orifice General signs of morbidity including one or more of the following: Depression, anorexia, marked change in fecal or urine output, ruffled fur, dehydration, hunched posture