Identification and characterization of 2-keto-3-deoxy-L
advertisement

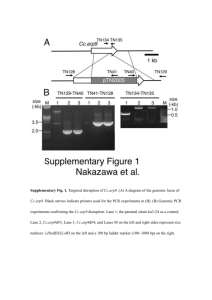
Supplementary materials Identification and characterization of 2-keto-3-deoxy-L-rhamnonate dehydrogenase belonging to the MDR superfamily from the thermoacidophilic bacterium Sulfobacillus thermosulfidooxidans: implications to L-rhamnose metabolism in archaea Jungdon Bae1, Suk Min Kim1, Sun Bok Lee1,2* Department of Chemical Engineering1, Graduate School of Engineering Mastership2, Pohang University of Science and Technology, San 31, Hyoja Dong, Pohang 790-784, Korea -1- Table S1. Characteristics of four Rha_NMP genes in Sulfobacillus thermosulfidooxidans AT-1 and the primers used for cloning Locus tag Sulth_3557 Sulth_3558 Sulth_3559 Sulth_3560 COG category COG1063 COG0179 COG4948 Annotation L-threonine 3dehydrogenase ureidoglycolate lyase COG1028 3-oxoacyl-[acylcarrier-protein] reductase Predicted protein function 2-keto-3-deoxy-Lrhamnonate dehydrogenase 2,4-diketo-3-deoxyL-rhamnonate hydrolase L-rhamnose dehydrogenase L-rhamnonate dehydratase Superfamily MDR fumarylacetoacetate hydrolase SDR ORF (bp) AA (ea) MW (Da) GC (%) 996 331 35,694 49.2 900 299 33,362 45.1 762 253 27,114 47.4 mandelate racemase/muconate lactonizing enzyme 1,194 397 44,500 45.4 GAGGATCCATGAA AACTTTAACATGG ACGGC CCAAGCTTCTAAA ATGTTAAGATGATT TTG BamHI/HindIII GAGGATCCATGAA ACTAGCCAGTGCC ATAG CCAAGCTTTCATG ACTTTGTGTTCGC T BamHI/HindIII GTTGGATCCATGG CATTAACAGGAAA AGTGG TATAAGCTTTCACT GTAAATTCACAAA CAATC BamHI/HindIII TTAGGATCCATGA AAAATAACCTGAA AATTGTTC CCCTGCAGCTAAG ACGGCGCAGAGG AAACG BamHI/PstI Primer (forward) Primer (reverse) Restriction sites Expression vector PCR L-rhamnonate dehydratase pQE-80L 33 cycles, denaturation at 94°C for 30 sec, annealing at 55°C for 40 sec, and polymerization at 72°C for 40 sec. -2- Figure S1. SDS-PAGE of recombinant proteins purified from the E. coli DH5α transformants. A. Sulth_3559 (St_RhaDH). B. Sulth_3557 (St_KDRDH). Lane M, Tech & Innovation ACCU prestained protein marker; lane 1, supernatant protein sample after sonication; lane 2, precipitated protein sample after sonication lane 3, protein sample purified by Ni-NTA affinity chromatography; lane 4, protein sample purified by Q-Sepharose FF chromatography. The amount of protein loaded onto a gel was 15 µg. The arrows indicate Sulth_3559 (A) and Sulth_3557 (B), respectively. -3- Figure S2. Kinetic parameter determination of the recombinant St_KDRDH. (A) Five different LKDR concentrations (0.1-5 mM) in the presence of 1 mM NAD+, (B) six different NAD+ concentrations in the presence of 1 mM L-KDR, (C) six different BDO concentrations (0.1-100 mM) in the presence of 1 mM NAD+, and (D) six different NAD+ concentrations in the presence of 50 mM BDO. Apparent Vmax and Km values were determined by the Lineweaver–Burk plot using SigmaPlot (version 10.0). Michaelis–Menten plots are shown in the inset. -4-










![Anti-Lactate Dehydrogenase antibody [98A-1F9BB1] ab135396](http://s2.studylib.net/store/data/011973894_1-4009b4c3b43d059b802b552d6fca31f6-300x300.png)