Visual/Auditory Activity
advertisement
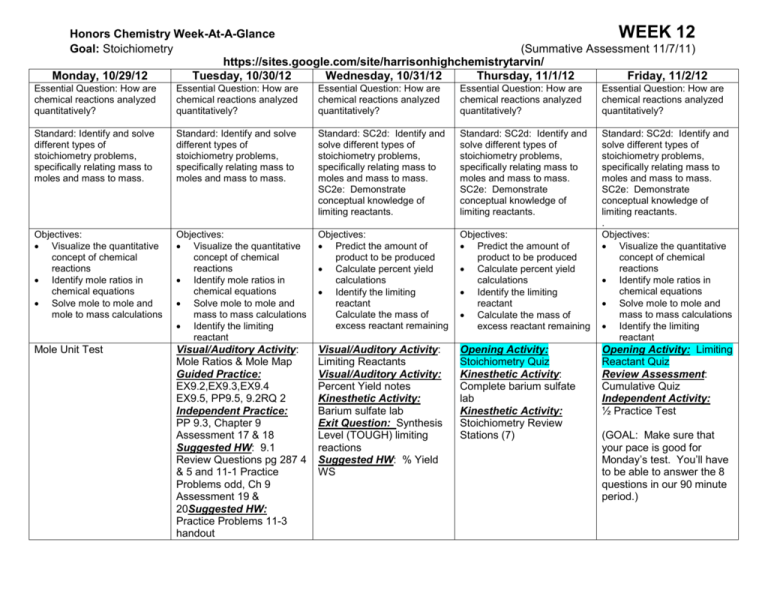
Honors Chemistry Week-At-A-Glance WEEK 12 Goal: Stoichiometry (Summative Assessment 11/7/11) https://sites.google.com/site/harrisonhighchemistrytarvin/ Monday, 10/29/12 Tuesday, 10/30/12 Wednesday, 10/31/12 Thursday, 11/1/12 Friday, 11/2/12 Essential Question: How are chemical reactions analyzed quantitatively? Essential Question: How are chemical reactions analyzed quantitatively? Essential Question: How are chemical reactions analyzed quantitatively? Essential Question: How are chemical reactions analyzed quantitatively? Essential Question: How are chemical reactions analyzed quantitatively? Standard: Identify and solve different types of stoichiometry problems, specifically relating mass to moles and mass to mass. Standard: Identify and solve different types of stoichiometry problems, specifically relating mass to moles and mass to mass. Standard: SC2d: Identify and solve different types of stoichiometry problems, specifically relating mass to moles and mass to mass. SC2e: Demonstrate conceptual knowledge of limiting reactants. Standard: SC2d: Identify and solve different types of stoichiometry problems, specifically relating mass to moles and mass to mass. SC2e: Demonstrate conceptual knowledge of limiting reactants. Objectives: Visualize the quantitative concept of chemical reactions Identify mole ratios in chemical equations Solve mole to mole and mole to mass calculations Objectives: Visualize the quantitative concept of chemical reactions Identify mole ratios in chemical equations Solve mole to mole and mass to mass calculations Identify the limiting reactant Objectives: Predict the amount of product to be produced Calculate percent yield calculations Identify the limiting reactant Calculate the mass of excess reactant remaining Objectives: Predict the amount of product to be produced Calculate percent yield calculations Identify the limiting reactant Calculate the mass of excess reactant remaining Standard: SC2d: Identify and solve different types of stoichiometry problems, specifically relating mass to moles and mass to mass. SC2e: Demonstrate conceptual knowledge of limiting reactants. . Objectives: Visualize the quantitative concept of chemical reactions Identify mole ratios in chemical equations Solve mole to mole and mass to mass calculations Identify the limiting reactant Mole Unit Test Visual/Auditory Activity: Mole Ratios & Mole Map Guided Practice: EX9.2,EX9.3,EX9.4 EX9.5, PP9.5, 9.2RQ 2 Independent Practice: PP 9.3, Chapter 9 Assessment 17 & 18 Suggested HW: 9.1 Review Questions pg 287 4 & 5 and 11-1 Practice Problems odd, Ch 9 Assessment 19 & 20Suggested HW: Practice Problems 11-3 handout Visual/Auditory Activity: Limiting Reactants Visual/Auditory Activity: Percent Yield notes Kinesthetic Activity: Barium sulfate lab Exit Question: Synthesis Level (TOUGH) limiting reactions Suggested HW: % Yield WS Opening Activity: Stoichiometry Quiz Kinesthetic Activity: Complete barium sulfate lab Kinesthetic Activity: Stoichiometry Review Stations (7) Opening Activity: Limiting Reactant Quiz Review Assessment: Cumulative Quiz Independent Activity: ½ Practice Test (GOAL: Make sure that your pace is good for Monday’s test. You’ll have to be able to answer the 8 questions in our 90 minute period.)