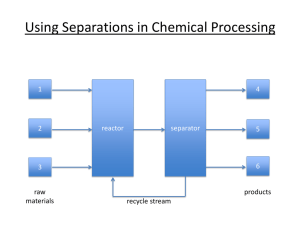
Course Prefix, Number, Title, Credit Hours: CBE, 464, Chemical
advertisement

Course Prefix, Number, Title, Credit Hours: CBE, 417, Chemical Engineering Equilibrium Separations, (2-0) 2 University Name: South Dakota School of Mines and Technology Academic Term, Year: Fall, 2012 Course Meeting Time and Location: 10:00 – 10:50 M, W, F, CB-106 Instructor’s Contact Information Name: David Dixon Office location: C-222 ½ Office hours: By appointment, or drop by at open schedule times Office phone number: 605-394-1235 Email address: david.dixon@sdsmt.edu Course Description Catalog description: The fifth course on the theory and practice of chemical engineering with emphasis on equilibrium staged separations. Additional course description (optional): Course Prerequisites Previous courses/experience: CBE 321 Technology skills: Students should have a working knowledge of material and energy balances and phase equilibrium calculations. It is helpful if they have an understanding of mass transfer in stagnant and turbulent systems and are familiar with absorber modeling and design in packed tower equipment. Students should be familiar with setting up and running basic AspenPlus blocks. Description of Instructional Methods: The main type of instructional method utilized in this class is classroom lecture augmented with in class exercises and homework. Communication technologies available in the classroom provided will be utilized. Course Requirements Required textbook(s) and other materials: Seader., J.D., E.J. Henley, and D.K. Roper, Separation Process Principles, 3rd ed., John Wiley & Sons, Inc., 2011. Supplementary materials: Perry, R.H., and D. Green, Chemical Engineers’ Handbook, 6th ed (or others as needed), McGraw-Hill, 1984. Wankat, Phillip C., Separation Process Engineering, 2nd ed., Prentice Hall, 2007. Seader, J.D., and E.J. Henley, Separation Process Principles, 2nd ed., Wiley, 2005. Geankoplis, C.J., Transport Processes and Separation Process Principles, 4th ed., Prentice Hall, 2003. Schweitzer, P.A., Handbook of Separation Techniques for Chemical Engineers, 3rd ed., McGraw-Hill, 1997. McCabe, W.L., J.C. Smith, and P. Harriott, Unit Operations of Chemical Engineering, 7th ed., McGraww-Hill, 2004. Treybal, R.E., Mass-Transfer Operations, 3rd ed., McGraw-Hill Book Company, 1980. Class attendance policy See current SDSMT catalog Cheating and plagiarism policy See current SDSMT catalog: Policy Governing Academic Integrity Make-up policy If a class meeting or deadline is missed due to pre-planned or unforeseen circumstances the instructor should be contacted as soon as possible to arrange for possible makeup work. Late work is not accepted for full credit, except under extenuating circumstances. Course Goals Include specific reference to System General Education Goals if applicable. Students in this course should expect to: Understand the phase equilibrium principles that govern staged chemical separation processes. Develop the ability to model separation processes using material and energy balances combined with phase equilibrium expressions (equilibrium stage approach). Understand the advantages and limitations of this approach. Numerical solutions may be obtained by hand, by spreadsheet, and using steady-state simulators (e.g., ASPEN RADFRAC). Obtain a practical, working knowledge of common separation operations such as distillation, absorption/stripping, and liquid-liquid extraction. Student Learning Outcomes: After completion of this course the average student is expected to be able to: 1. Understand the physical principles and basic mechanical designs of fundamental separation operations (flashes, distillation, absorption-stripping, and liquid-liquid extraction). 2. Develop an equilibrium stage model of a separation process from first principles (material and energy balances, combined with phase equilibrium calculations). 3. Use various mathematical tools (hand calculations, graphical solutions, math software, and ASPEN software) to simultaneously solve the material balances, energy balances, and phase equilibrium expressions needed to model equilibrium-staged separations. 4. Use equilibrium stage model solutions to design and troubleshoot separation operations. 5. Combine equilibrium stage models with simple cost estimates to optimize separation designs from an economic standpoint. Evaluation Procedures Assessments Tests, projects, assignments, etc. The final course grade will be the result of evaluation of a combination of instruments that could include; tests, quizzes, homework, design projects, and participation in a team. 100 points in the course are assigned the following distribution: three exams (include a comprehensive final exam); 20 pts each 60 pts homework and possible quizzes; 30 pts team project 10 pts Performance standards/grading policy Final grading will be by letter grades according to the following percentages: 90-100 =>A; 80-89 => B; 70-79 => C; 60-69 => D; <60 => F. Homework and quizzes are used to reinforce learning of the material and to allow assessment of understanding of lecture material, reading assignments, and homework problems. Homework is due at the start of each class period when it is requested. Homework to be graded will be chosen at random. Work should be done professionally. From time to time homework may be assigned to be done and turned in as a group. Other homework can be worked on in group study sessions, however individual solutions are to be turned in. Teams and individual efforts. A team project will be assigned to a group (team) of 3-5 members. It is important for each member of the team to participate actively and to contribute meaningfully to the team’s project work. Full credit is given to those members who do so. ADA Statement Students with special needs or requiring special accommodations should contact the instructor, (David Dixon, at 605-394-1235) and/or the campus ADA coordinator, Jolie McCoy, at 394-1924 at the earliest opportunity. Freedom in Learning Statement Freedom in learning. Under Board of Regents and University policy student academic performance may be evaluated solely on an academic basis, not on opinions or conduct in matters unrelated to academic standards. Students should be free to take reasoned exception to the data or views offered in any course of study and to reserve judgment about matters of opinion, but they are responsible for learning the content of any course of study for which they are enrolled. Students who believe that an academic evaluation reflects prejudiced or capricious consideration of student opinions or conduct unrelated to academic standards should contact the dean of the college which offers the class to initiate a review of the evaluation. Electronic Devices Policy Professional decorum. Tentative Course Outline/Schedule Note, this is a tentative list of topics and is subject to change depending on the class needs and the instructor. All topics in the chapters may not be covered and some may be covered to a greater depth than others. Single stage vapor-liquid equilibrium flash Cascade Basic principles of distillation McCabe-Theile diagrams Trayed tower design, including reboilers and condensers Tray efficiencies in distillation Shortcut methods for multicomponent distillation Rigorous distillation modeling using RADFRAC in AspenPlus Complex distillation Ternary diagrams for single stage liquid-liquid extraction Multistage countercurrent liquid-liquid extraction Additional Course Information RELATION OF COURSE OUTCOMES TO PROGRAM OUTCOMES (2011 - ): The following table indicates the relative strengths of each course outcomes in addressing the program outcomes (on a scale of 1 to 4 where 4 indicates a strong emphasis) Course Outcomes CBE 417 (1) (2) (3) (4) (5) Outcome 1 a b 4 4 4 4 Outcome 2 a b ChE Program Outcomes* Outcome 3 Outcome 4 a b a b 3 Outcome 5 a b Outcome 6 a b 4 3 *For a list of Program Objectives and Program Outcomes, please go to: http://cbe.sdsmt.edu/undergraduate Revised Aug 2007 from BOR June 2004