120712ChE128-1-EquilStages
advertisement
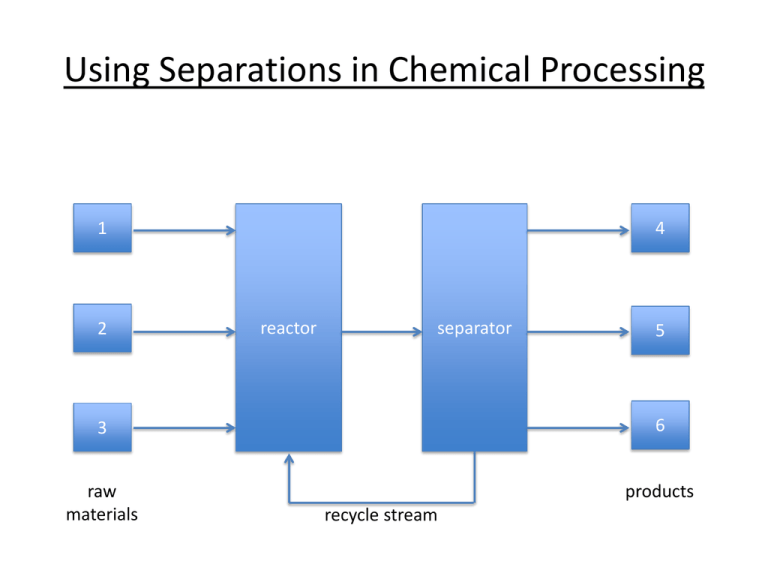
Using Separations in Chemical Processing 1 2 4 reactor separator 5 3 6 raw materials products recycle stream Where are separations needed? • Purification of reactor feeds • Purification of products for sale • Purification of waste for safe disposal Separations as Unit Operations • The specific design of the separator depends on the chemical composition of the feed, and the desired purity of the product • However, the general design principals are independent of the chemistry Column distillation At an oil refinery, fractional distillation columns separate hydrocarbons into separate streams, cuts or fractions A multi-purpose distillation column for mineral oils and chemicals 40 trays with multiple feed entry options, capacity: up to 45 mt/h mode: vacuum, atmospheric or pressure up to 3 bar temperature: up to 320°C Flash vaporization Flash drum for hydrocarbon vapor recovery Water desalination plant in Cyprus Multistage flash distillation Absorption and stripping A column filled with an amine solution is used to absorb H2S and CO2 from “sour” natural gas A steam stripping column removes H2S and CO2 to regenerate the amine Liquid-liquid extraction Mixer-settlers used for continuous, counter-current liquid-liquid extraction of rare-earth ions Leaching Cyanide leaching of gold ore, Nevada Sublimation Sublimation of HgI2 for use in semiconductor manufacturing, as well as in detectors for X-ray and g-ray imaging Crystallization Multiple-Effect Crystallizer for Sodium Sulfate (Na2SO4) Refining Crystallizer for Salt (NaCl) Chromatography Chromatography columns Membrane filtration Water Treatment Plant. Each white vessel contains seven spiral-wound membrane units. Why is good design important for separations? • Separations equipment can be 50-90 % of the capital investment in a chemical plant • Separations can also represent 40-70 % of operating costs • Purity requirements depend on market tolerance – High separations costs tolerated for high valueadded products Examples 1. Petroleum refining crude oil gasoline, diesel, jet fuel, fuel oil, waxes, coke, asphalt 2. Pharmaceuticals sub-ppm level of metal catalyst required for human consumption 3. Semiconductors SiO2 SiCl4 Si Metallurgical grade (97%) for alloying with steel and Al: $1/kg Solar grade for photovoltaics (99.99 %): $80/kg 4. Water treatment Industrial wastewater vs. potable water Some impurities ok (Ca2+); others not (Hg2+) Process Diagram for Ethylene hydration: C2H4 + H2O C2H5OH Why do separations cost a lot? • “unmixing” causes reduction in entropy • this is not spontaneous • achieve by adding an external separating agent – – – – Energy (distillation) Material (e.g. extraction) Barrier (e.g. membrane) Gradient (e.g. electrophoresis) Table 1. Separation Unit Operations based on Phase Creation or Addition vertical drum horizontal drum valve column with trays (stages) heat exchangers condensor reboilers Table 1, cont. Separation Unit Operations Based on Phase Creation or Addition heater Table 1, cont. Separation Unit Operations Based on Phase Creation or Addition Table 2. Separation Unit Operations based on a Solid Separating Agent Table 3. Separation Unit Operations Based on the Presence of a Barrier Table 4. Separation Unit Operations Based on an Applied Field or Gradient Equilibrium-staged separations • Make use of thermodynamics to achieve spontaneous separation • But thermodynamics also dictates the limits of the separation Definitions of equilibrium vapor thermal equilibrium: Tliq = Tvap mechanical equilibrium: Pliq = Pvap liquid chemical equilibrium: mliq = mvap (chemical potential) Equilibrium is dynamic: molecules continue to vaporize and condense, but at equal rates, so there is no net change in either phase. Rate of approach to equilibrium depends on: (1) rate of mass transfer proportional to (a) mass transfer coefficients Ki = f(T), and (b) interfacial area (2) concentration gradient becomes very small as equilibrium is approached, ∞ time required to achieve Consider a single equilibrium stage • V and L are in equilibrium with each other; they are streams leaving the same equilibrium stage. • V and L are not in equilibrium with F, i.e., miL = miV ≠ miF vapor product flow rate V T, P composition yi T, P feed vapor flow rate F TF, PF composition zi liquid liquid product • if > 1 chemical species present, then xi ≠ yi • therefore separation has occurred • vapor-liquid equilibrium (VLE) limits the amount of separation that can be achieved flow rate L T, P composition xi 25 Cascade of equilibrium stages What if we need more separation than one equilibrium stage can provide? Feed one of the two product streams (e.g., L) to another equilibrium stage V 1 F V2 stage 1 V3 stage 2 L 1 L2 stage 2 L3 • Creates many vapor streams with different compositions • If we combine (mix) them, we destroy some of the separation we created • If we discard them, our yield is low. Better Alternative: Counter-current cascade F Variable pressure cascade P1 > P2 > P3 1 L1 V2 2 L2 V1 1 L1 compressor • replace • replace V2 by 2 by L2 V3 3 L3 F V1 Variable temperature cascade T1 > T2 > T3 V3 3 L3 An even better alternative F V1 Integrate the heat exchangers: allow contact between condensing vapor and vaporizing liquid streams F V1 1 liquid downcomer L1 V2 2 L2 weir V3 perforated tray 1 V2 L1 2 V3 L2 3 vapor 3 L3 L3 • integrated column is isobaric and non-isothermal • promotes mixing of liquid and vapor phases Thermodynamic considerations • Perfect separation requires an infinite number of equilibrium stages • The engineer specifies the number of stages required for an acceptable degree of separation • Equilibrium is not achieved on each stage in a finite time theoretical stage: assume equilibrium is achieved actual stage: equilibrium is not achieved (< 100 % efficiency) • We always need more than the theoretical number of stages to achieve the desired separation • The engineer’s role is to decide how many more General design procedure for equilibrium-staged separations 1. Obtain relevant equilibrium data (where?) * 2. Determine no. of theoretical stages required 3. Determine no. of actual stages required (requires knowledge of stage efficiency) 4. Size equipment, based on expected flow rates F, V, L * Focus of this course.









