SOL Review – May 6, 2013 Topic: Acid/Base Theory Arrhenius
advertisement

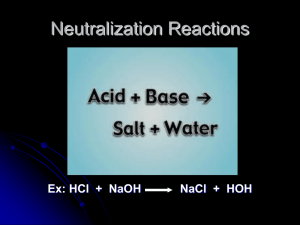
SOL Review – May 6, 2013 Topic: Acid/Base Theory Arrhenius Theory: Acids: substances that produce H+ ions in solution Examples: HCl, H2SO4, HNO3 Bases: substances that produce OH- ions in solution Examples: NaOH, Ca(OH)2, KOH Neutralization reaction: When an acid is added to a base, the products are water and a salt. Examples: NaOH + HCl H2O + NaCl HNO3 + Ca(OH)2 H2O + Ca(NO3)2 Bronsted-Lowry Theory: Acids: substances that donate a proton (H+) Bases: substances that accept a proton (H+) Example: HC2H3O2 + NH3 C2H3O2- + NH4+ 1 pH Scale: H+ ion concentration (M) 1 10-1 10-2 10-3 10-4 pH value 0 1 2 3 4 10-5 5 10-6 10-7 10-8 10-9 10-10 10-11 10-12 10-13 10-14 6 7 8 9 10 11 12 13 14 pH pH = -log[H+] [H+] = 10-pH [H+] OH- ion concentration (M) 10-14 10-13 10-12 10-11 10-10 Examples Hydrochloric acid Stomach acid Lemon juice Vinegar, Coca-Cola, Beer Tomatoes Black Coffee, Normal Rainwater Urine Pure water, Blood, Tears Seawater Baking soda Great Salt Lake Household Ammonia Bicarbonate of Soda Oven cleaner Sodium Hydroxide pH + pOH = 14 10-8 10-7 10-6 10-5 10-4 10-3 10-2 10-1 1 pOH [OH-] = 10-pOH Kw = [H+][OH-] Kw = 1.0 x 10-14 10-9 pOH = -log[OH-] [OH-] 2 Examples: 1. If the [H+] is 1.5 x 10-3 M, what is the [OH-]? 𝐾𝑤 = [𝐻 + ][𝑂𝐻 − ] 𝐾𝑤 = [𝑂𝐻 − ] [𝐻 + ] (1.0𝑥10−14 ) = [𝑂𝐻 − ] = 6.7𝑥10−12 𝑀 (1.5𝑥10−3 ) 2. If the [H+] = 4.3 x 10-6 M, what is the pH? 𝑝𝐻 = − log[𝐻 + ] 𝑝𝐻 = − log(4.3𝑥10−6 ) 𝑝𝐻 = 5.37 3. If the [H+] = 7.8 x 10-10 M, what is the pOH? 𝑝𝐻 = − log[𝐻 + ] 𝑝𝐻 = − log(7.8𝑥10−10 ) = 9.11 𝑝𝐻 + 𝑝𝑂𝐻 = 14 14 − 𝑝𝐻 = 𝑝𝑂𝐻 = 14 − 9.11 = 𝑝𝑂𝐻 = 4.89 4. If the pH is 5.61, what is the [OH-]? 𝑝𝐻 + 𝑝𝑂𝐻 = 14 14 − 𝑝𝐻 = 𝑝𝑂𝐻 = 14 − 5.61 = 𝑝𝑂𝐻 = 8.39 [𝑂𝐻 − ] = 10−𝑝𝑂𝐻 [𝑂𝐻 − ] = 10−8.39 = 4.07𝑥10−9 𝑀 3 Titration Process: A titration is an experimental process that is used to determine the concentration of substance, most often an acid or a base. A buret is used to add a specific amount of the titrant to the solution that is being analyzed, the analyte. An indicator is used to show the exact point when the analyte is neutralized by the titrant. An indicator is a substance that changes color depending on the pH of the solution it is in. The most common indicator is phenolphthalein. Phenolphthalein is fuchsia in basic solutions and clear in acidic solutions. Another way to determine the endpoint of a titration is by using litmus paper. There are two types of litmus paper: red litmus paper and blue litmus paper. Red litmus paper turns blue when it is exposed to a basic solution and stays red when it is exposed to an acidic solution. Blue litmus paper turns red when it is exposed to an acidic solution and stays blue when it is exposed to a basic solution. At the endpoint of the titration (at the neutralization point), the moles of the acid are equal to the moles of the base. By knowing this relationship, you can determine the concentration of the analyte. You can use the formula, M1V1 = M2V2 -This formula can only be used when the ratio of the acid to the base is 1:1 So for example, 25mL of a 1.5M solution of NaOH were used to completely neutralize 15mL of HCl. What is the concentration of the HCl solution? M1 = 1.5M NaOH M2 = ? M HCl M1V1 = M2 V2 V1 = 25mL NaOH V2 = 15mL HCl (1.5M)(25mL) = M2 = 2.5M HCl (15mL) Another example: 16.43mL of a 0.100M solution of HBr were used to completely neutralize 14.55mL of KOH. What is the concentration of the basic solution? M1 = 0.100M HBr M2 = ? M KOH M1V1 = M2 V2 V1 = 16.43mL HBr V2 = 14.55mL KOH (0.100M)(16.43mL) = M2 = 0.113M KOH (14.55mL) 4












![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)