Scientific Skills Review-key
advertisement

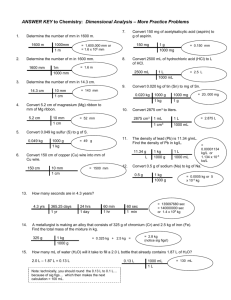
Scientific Skills Review2012-Key Name:____________________________________ 1. A student set-up an experiment to determine if the addition of sugar to bubble mix would affect the amount of bubbles produced. a. b. What is the independent variable? Independent: “I “ control: concentration of sugar to bubble mix What is the dependent variable? Amount of bubbles produced What could be a quantitative and qualitative observation from this experiment? Qualitative: observe amount of bubbles produced Quantitative: measure amount of bubbles produced c. 2. Students measured the mass of an igneous rock three different times and recorded their values below. The actual value for the igneous rock is 25.233g. Matter Trial #1 (g) Trial #2 (g) Trial #3 (g) Avg. Mass Igneous Rock 25.232 25.234 25.231 25.232 g a. Is her data precise? Yes, the expt. trials are close to one another. How accurate is her data? Very accurate, just 0.004 % error. Use the calculation below to answer this question. (experimental value) − (actual value) % error = ――――――――――――― actual value × 100 Remember absolute value applies prior to converting to percent. = 0.004 % error, therefore 99.996 % accurate 3. Convert each of the following data to scientific notation. a. The diameter of a human hair is approximately 0.0000008m. = 8.0x10-7 m b. It is estimated that there is around 300 billion stars in our galaxy. = 3x1011 stars c. A nuclear bomb’s temperature at the epic center has been recorded to be 300,000 0C. = 3x105 oC d. The density of oxygen is 0.001429 g/cm3 at room temperature. = 1.429x10-3 g/cm3 3. Convert each of the following data to standard notation. a. A small gold nugget has a mass of 3.40x10-2 m on each edge. = 0.0340 m b. The volume of the earth is 1.08321x1012 km3. = 1,083,210,000,000 km3 c. A gram of hydrogen consists of 6.02 x 1023 atoms. = 602,000,000,000,000,000,000,000 atoms d. A viral germ can be as small as 2.0x10-10 m. (Always wash hands before eating!) = 0.0000000002 m 4. Metric to Metric Conversions: a. 500kg = ____50,000,000_____cg b. 1.5dL = _150________mL c. 330ms = _0.330_________s d. 14.9 mm = _0.00149_________dkm 5. Determine the number of significant figures in each measurement below. a. 2.004 g = 4 b. 0.120 mg = 3 c. 120 mL =2 d. 120.00 mL=5 e. 1.43x1034 km = 3 6. Calculate each answer with the correct number of significant figures. a. 1.0g x 0.010 g = 0.010 g2 b. 1.210 + 1.4g + 3.0g = 5.6 g c. 0.132 mL - 0.002mL = 0.130 mL d. 8,000g / 225g = 40 7. Complete calculations using correct number of significant figures. Apply calculation rules to measurements only, NOT conversion factors Ex. 16 oz. = 1 lb. If multiple mathematical operations are involved in getting answer, the last operation determines what calculation rule needs to be applied so that the answer is expressed to the right degree of accuracy. a. A basketball weighs 22 oz. How many milligrams is that equivalent to? (oz. --> lbs.--> g ---> mg) = 620,000 mg (2 sig. figs) b.The sun is 1.496x108 km away from earth. How many miles is that equivalent to? (km-- mi.) = 92,750,000 mi. (4 sig. figs) c.A farmer has 135 acres for his cattle to graze on. How many square feet is that equivalent to? (one acre= 43,560 ft2) ( acre - ft2) = 5,880,000 ft2 (3 sig. figs) d.A 100.0 mL sample of helium has a mss of 0.1785g. What is the density of helium? Density = mass/volume = 0.001785g/mL (4 sig. figs) e. If a 48.55 g sample of iron has a density of 0.971 g/cm3 what would its volume in cm3 be? 50.0 cm3 (3 sig. figs) f. Hydrogen has a boiling point of 20.13 Kelvins (K). What would that be equivalent to in degrees Celsius? ( K = 0C + 273) = -252.87 oC (2 places past decimal) g. In 2006 along with creating 250 million tons of trash Americans did recycle 80 million tons of waste. Determine how many grams of waste were recycled. t lbs - g = 7x1013 g (1 sig. fig) h. The volume of a soccer ball is 5.425x10-1 cm3. How many dL is that equal to? cm3 - mL - dL = 0.005425 dL i. A cube of gold-colored metal with a volume of 59 cm3 has a mass of 980 grams. The density of pure gold is 19.3 g/cm3. Is the metal pure gold? Show calculations to validate answer. density of cube = 17g/cm3 (2 sig. figs) No, not pure gold. j. A student is curious to observe what the boiling point of chlorine is. She performs the experiment three times and records the following results: Experimental Trials Boiling Point of Chlorine 0C 1 -33.0 2 -33.3 3 -33.2 The actual boiling point of chlorine is known to be 239.15 Kelvin. = -33.85 oC * Is her experimental data precise? Yes, the expt. trials are very close. *Is her experimental data accurate? Avg. for expt. data= -33.2 oC *Calculate how accurate her data is using the percent error equation. % error = 1.92 % error, therefore 98.08% accurate k. 8. If you ran a marathon, how many meters would that be equivalent to? A marathon is 26.3 miles. mi km - m 42,400 m (3 sig. figs) A student is performing a science fair project called “Does temperature affect how high a basketball can bounce? a. What is the independent and dependent variable? ID = temperature D: height ball bounces b. Identify a qualitative and quantitative observation from this experiment. qualitative: observe height of ball quantitative: measure height of ball c. What are some factors he needs to keep constant. Type of ball, height ball dropped, where ball dropped d. Explain what your hypothesis would be for this experiment.