(Vd)=(.75L) 1.56x10-2L KClO3 150g 190g unsaturated
advertisement
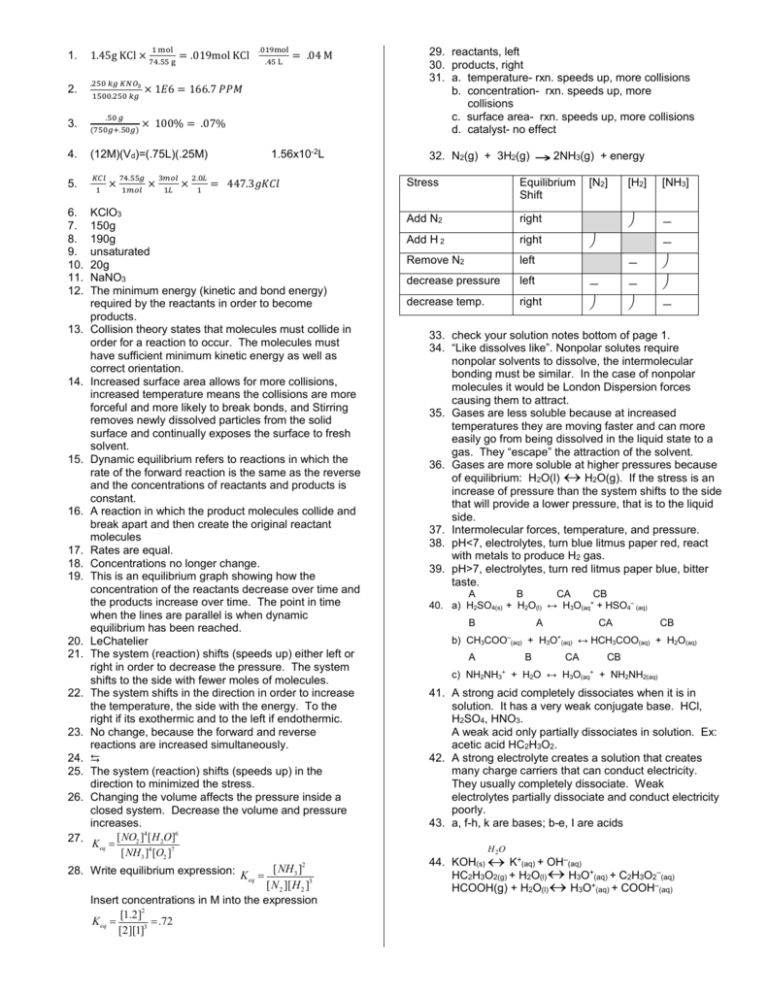
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 1.45g KCl × .250 𝑘𝑔 𝐾𝑁𝑂3 .50 𝑔 = .019mol KCl 1 × .45 L = .04 M × 100% = .07% (12M)(Vd)=(.75L)(.25M) 𝐾𝐶𝑙 .019mol × 1𝐸6 = 166.7 𝑃𝑃𝑀 1500.250 𝑘𝑔 (750𝑔+.50𝑔) 1 mol 74.55 g 74.55𝑔 1𝑚𝑜𝑙 × 3𝑚𝑜𝑙 1𝐿 × 2.0𝐿 1 1.56x10-2L = 447.3𝑔𝐾𝐶𝑙 KClO3 150g 190g unsaturated 20g NaNO3 The minimum energy (kinetic and bond energy) required by the reactants in order to become products. Collision theory states that molecules must collide in order for a reaction to occur. The molecules must have sufficient minimum kinetic energy as well as correct orientation. Increased surface area allows for more collisions, increased temperature means the collisions are more forceful and more likely to break bonds, and Stirring removes newly dissolved particles from the solid surface and continually exposes the surface to fresh solvent. Dynamic equilibrium refers to reactions in which the rate of the forward reaction is the same as the reverse and the concentrations of reactants and products is constant. A reaction in which the product molecules collide and break apart and then create the original reactant molecules Rates are equal. Concentrations no longer change. This is an equilibrium graph showing how the concentration of the reactants decrease over time and the products increase over time. The point in time when the lines are parallel is when dynamic equilibrium has been reached. LeChatelier The system (reaction) shifts (speeds up) either left or right in order to decrease the pressure. The system shifts to the side with fewer moles of molecules. The system shifts in the direction in order to increase the temperature, the side with the energy. To the right if its exothermic and to the left if endothermic. No change, because the forward and reverse reactions are increased simultaneously. ⇆ The system (reaction) shifts (speeds up) in the direction to minimized the stress. Changing the volume affects the pressure inside a closed system. Decrease the volume and pressure increases. [NO2 ]4 [H 2O]6 Keq = [NH 3 ]4 [O2 ]7 2 Write equilibrium expression: K = [NH 3 ] eq [N 2 ][H 2 ]3 Insert concentrations in M into the expression [1.2]2 K eq = = .72 [2][1]3 29. reactants, left 30. products, right 31. a. temperature- rxn. speeds up, more collisions b. concentration- rxn. speeds up, more collisions c. surface area- rxn. speeds up, more collisions d. catalyst- no effect 32. N2(g) + 3H2(g) 2NH3(g) + energy Stress Equilibrium Shift Add N2 right Add H 2 right Remove N2 left decrease pressure left decrease temp. right [N2] ¯ [H2] [NH3] ¯ ¯ ¯ - ¯ ¯ 33. check your solution notes bottom of page 1. 34. “Like dissolves like”. Nonpolar solutes require nonpolar solvents to dissolve, the intermolecular bonding must be similar. In the case of nonpolar molecules it would be London Dispersion forces causing them to attract. 35. Gases are less soluble because at increased temperatures they are moving faster and can more easily go from being dissolved in the liquid state to a gas. They “escape” the attraction of the solvent. 36. Gases are more soluble at higher pressures because of equilibrium: H2O(l) « H2O(g). If the stress is an increase of pressure than the system shifts to the side that will provide a lower pressure, that is to the liquid side. 37. Intermolecular forces, temperature, and pressure. 38. pH<7, electrolytes, turn blue litmus paper red, react with metals to produce H2 gas. 39. pH>7, electrolytes, turn red litmus paper blue, bitter taste. A B CA CB 40. a) H2SO4(s) + H2O(l) ↔ H3O(aq+ + HSO4– (aq) B b) c) A CA CH3COO–(aq) + H3O A B NH2NH3+ + + H2O ↔ (aq) CB ↔ HCH3COO(aq) + H2O(aq) CA H3O(aq+ CB + NH2NH2(aq) 41. A strong acid completely dissociates when it is in solution. It has a very weak conjugate base. HCl, H2SO4, HNO3. A weak acid only partially dissociates in solution. Ex: acetic acid HC2H3O2. 42. A strong electrolyte creates a solution that creates many charge carriers that can conduct electricity. They usually completely dissociate. Weak electrolytes partially dissociate and conduct electricity poorly. 43. a, f-h, k are bases; b-e, I are acids H 2O 44. KOH(s) « K+(aq) + OH–(aq) HC2H3O2(g) + H2O(l) « H3O+(aq) + C2H3O2–(aq) HCOOH(g) + H2O(l) « H3O+(aq) + COOH–(aq) 45. Base I– SO32– PO43– C2H3O2– Conjugate Acid HI_____ __HSO3–_ __HPO42-_ _ HC2H3O2_ Acid HClO4 H2S HCO3– H2SO4 Conjugate Base ___ClO4–_ ___HS–__ ___CO32-_ ___HSO4–_ 46. 2HClaq) + Mg(OH)2(aq) MgCl2(aq) + 2H2O(l) H2CO3(aq) + 2KOH(aq) K2CO3(aq) + 2H2O(l) H3PO4(aq) + 3NaOH(aq) Na3PO4(aq) + 3H2O(l) 47. Acid strength is measured using pH and it’s related to the % dissociation of the acid. Acid concentration is measured using molarity and it’s the # of moles per Liter. 48. What remains of the base after it has accepted a hydrogen ion from the acid. 49. What remains of the acid after it has donated it’s hydrogen ion to the base. 50. H2O(l) + H2O(l) « H3O(aq) + OH–(aq) 51. Keq=1.0x10-14 = [H+] [OH–] 52. pH = -log[1x10-5] =5 53. 1x10-14=[H+][OH-] 1x10-14=[H+][1x10-12] solve for H+ [H+]= .01M, use pH equation pH=-log[.01] =2 54. pH=-log[8.5 x 10-3] = 2.07 55. You could solve like question 53 or solve for pOH first, then use pH +pOH=14. pOH=-log[2.8x10-12] = 11.55 pH + 11.55=14 2.45 56. 12.75=-log[H+], multiply both sides by (-1), take inverse log of both sides. pH= 1.78 57. use: 11.63 + pOH=14 pOH=2.37, next use: pOH=-log[OH–] 2.37=-log[OH–], solve for OH– = 4.3x10–3M












![1. Find the pH of 0.1 M HClO4 This is a strong acid, so [H ] = 0.1 M](http://s3.studylib.net/store/data/008121755_1-338138652fc42091377fe33aaddd7c71-300x300.png)






