This requires 2 conversion factors!
advertisement
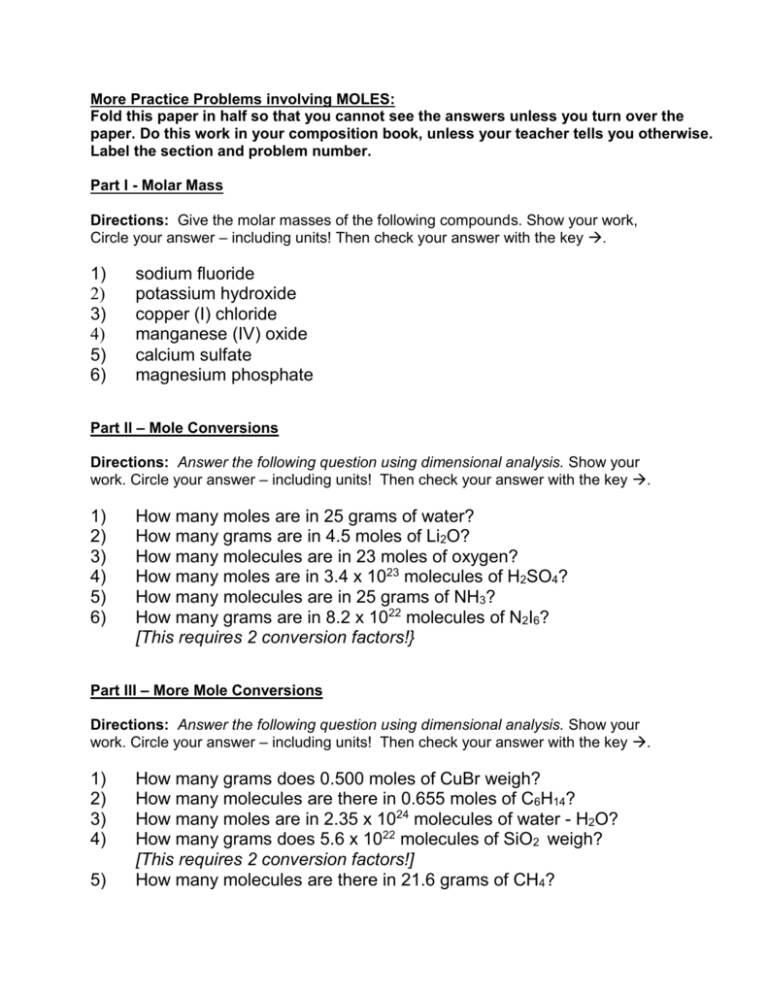
More Practice Problems involving MOLES: Fold this paper in half so that you cannot see the answers unless you turn over the paper. Do this work in your composition book, unless your teacher tells you otherwise. Label the section and problem number. Part I - Molar Mass Directions: Give the molar masses of the following compounds. Show your work, Circle your answer – including units! Then check your answer with the key . 1) 2) 3) 4) 5) 6) sodium fluoride potassium hydroxide copper (I) chloride manganese (IV) oxide calcium sulfate magnesium phosphate Part Il – Mole Conversions Directions: Answer the following question using dimensional analysis. Show your work. Circle your answer – including units! Then check your answer with the key . 1) 2) 3) 4) 5) 6) How many moles are in 25 grams of water? How many grams are in 4.5 moles of Li2O? How many molecules are in 23 moles of oxygen? How many moles are in 3.4 x 1023 molecules of H2SO4? How many molecules are in 25 grams of NH3? How many grams are in 8.2 x 1022 molecules of N2I6? [This requires 2 conversion factors!} Part IlI – More Mole Conversions Directions: Answer the following question using dimensional analysis. Show your work. Circle your answer – including units! Then check your answer with the key . 1) 2) 3) 4) 5) How many grams does 0.500 moles of CuBr weigh? How many molecules are there in 0.655 moles of C6H14? How many moles are in 2.35 x 1024 molecules of water - H2O? How many grams does 5.6 x 1022 molecules of SiO2 weigh? [This requires 2 conversion factors!] How many molecules are there in 21.6 grams of CH4? Part I - Molar Mass – ANSWERS 1) 2) 3) In sodium fluoride, there is one atom of sodium and one atom of fluorine. The molar mass will then be: (1 atom x 23 grams/mole of sodium) + (1 atom x 19 grams/mole of fluorine) = 42 grams/mole of sodium fluoride In potassium hydroxide, there is one atom of potassium, one atom of hydrogen, and one atom of oxygen. The molar mass will then be (1 x 39 grams) + (1 x 1 gram) + (1 x 16 grams) = 56 grams/mole of potassium hydroxide In copper (I) chloride, there is one atom of copper and one atom of chlorine. The molar mass is then (1 x 63.5 grams) + (1 x 35.5 grams) = 99 grams/mole of copper (I) chloride 4) In manganese (IV) oxide, there is one atom of manganese and two atoms of oxygen. The molar mass is then (1 x 55 grams) + (2 x 16 grams) = 87 grams/mole of manganese (IV) oxide 5) In calcium sulfate, there is one atom of calcium, one atom of sulfur, and four atoms of oxygen. The molar mass is then (1 x 40 grams) + (1 x 32 grams) + (4 x 16 grams) = 136 grams/mole of calcium sulfate 6) In magnesium phosphate, there are three atoms of magnesium, two atoms of phosphorus, and eight atoms of oxygen. (The formula is Mg3(PO4)2). The molar mass is then (3 x 24 grams) + (2 x 31 grams) + (8 x 16 grams) = 262 grams/mole of magnesium phosphate Part Il – Mole Conversions - ANSWERS 1) 2) 3) 4) 5) 6) 1.39 moles 134.1 grams 1.38 x 1025 molecules 0.56 moles 8.85 x 1023 molecules 106.7 grams Part Ill – More Mole Conversions - ANSWERS 1) 2) 3) 4) 5) 31.8 grams 3.94 x 1023 molecules 3.90 moles 5.59 grams 8.13 x 1023 molecules









