File
advertisement
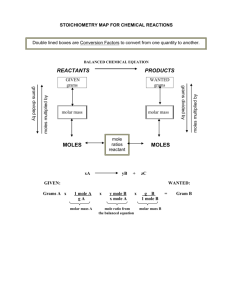
CHEMISTRY STOICHIOMETRY PANCAKE STOICHIOMETRY CALCULATORS REQUIRED PERIODIC TABLE REQUIRED THE KEY TO STOICHIOMETRY – THE MOLE RATIO • 2Al2O3(l) 4Al(s) + 3O2(g) • Mole ratios allow us to “convert” between different reactants and products. • What is the mole ratio between Al2O3 and Al? • 2 mol Al2O3 : 4 mol Al • We can write this as a conversion factor: 2m olAl2O3 4m olAl • We can now “convert” between amounts of different compounds. ONE CONVERSION: MOL MOL • CO2(g) + 2LiOH(s) Li2CO3(s) + H2O(l) • How many moles of lithium hydroxide are required to react with 20 mol CO2? • Molar ratio between CO2 and LiOH? • 1:2 YOU TRY: MOL MOL • How many moles of chlorine gas (Cl2) would react with 5 moles of sodium (Na) according to the following chemical equation? • 2 Na + Cl2 2 NaCl MASS-MASS STOICHIOMETRY • We just converted between moles of one substance into moles of another substance using a chemical reaction. • In practice, it is more common to convert from grams of one substance to grams of another. • We have to use the mole as a bridge. • How do we convert between grams and moles? STOICHIOMETRIC SETUP •A 2B • In order to convert between grams of A and grams of B, you have to use molar masses and the molar ratio as conversion factors. • If you are not given a reaction, you must generate it yourself! • grams A moles A moles B grams B (molar mass A) (molar ratio) (molar mass B) • It is very important to analyze the problem to figure out how many steps to use! • You can start and end at any point on this bridge!! SAMPLE PROBLEM 2 • What number of moles of O2 are needed to produce 14.2 grams of P4O10 from P? (molar mass of P4O10 = 284 g/mol) P + O2 P4O10 (unbalanced) SAMPLE PROBLEM 3 • 2NaCl 2Na + Cl2 • How many grams of chlorine gas are produced if 60 grams of sodium chloride are exposed to an electric current?