Periodic Trends – Ions and Ionic Radius
Name _________________________________ Hr____
How does the radius of an atom change when it becomes an ion?
Model 1
(Reference: Modern Chemistry pg. 153)
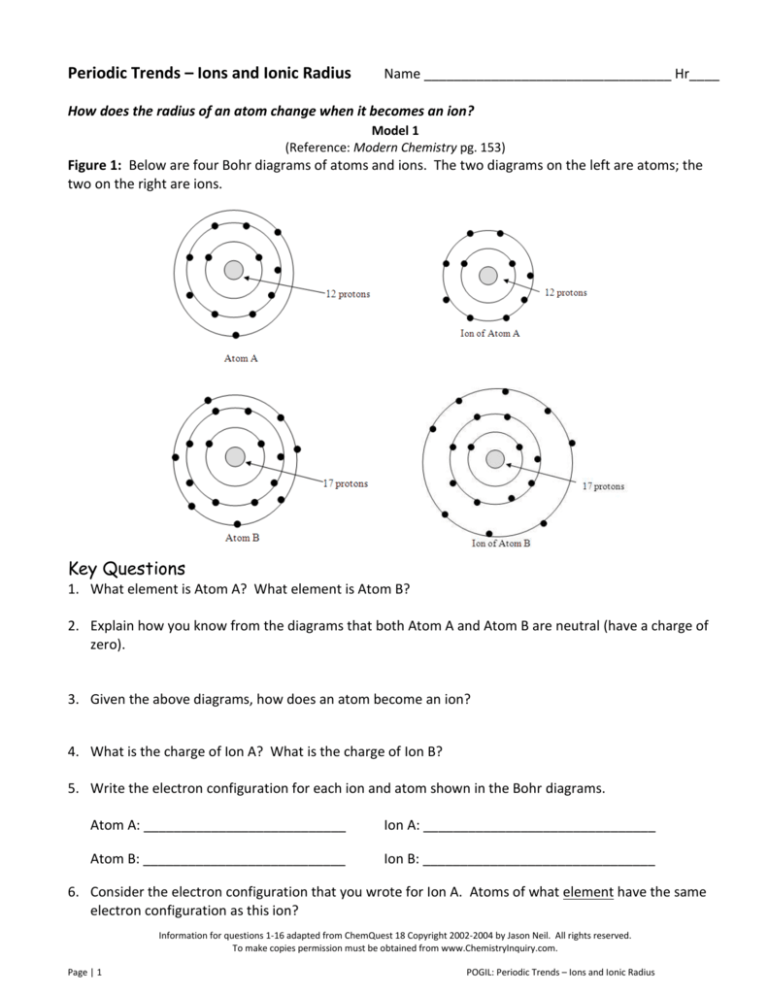
Figure 1: Below are four Bohr diagrams of atoms and ions. The two diagrams on the left are atoms; the
two on the right are ions.
Key Questions
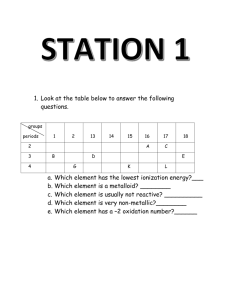
1. What element is Atom A? What element is Atom B?
2. Explain how you know from the diagrams that both Atom A and Atom B are neutral (have a charge of
zero).
3. Given the above diagrams, how does an atom become an ion?
4. What is the charge of Ion A? What is the charge of Ion B?
5. Write the electron configuration for each ion and atom shown in the Bohr diagrams.
Atom A: ___________________________
Ion A: _______________________________
Atom B: ___________________________
Ion B: _______________________________
6. Consider the electron configuration that you wrote for Ion A. Atoms of what element have the same
electron configuration as this ion?
Information for questions 1-16 adapted from ChemQuest 18 Copyright 2002-2004 by Jason Neil. All rights reserved.
To make copies permission must be obtained from www.ChemistryInquiry.com.
Page | 1
POGIL: Periodic Trends – Ions and Ionic Radius
7. Consider the electron configuration that you wrote for Ion B. Atoms of what element have the same
electron configuration as this ion?
8. Bromine atoms always gain one electron when they become an ion. Atoms of what element have the
same number of electrons as a bromine ion?
9. Cesium atoms always lose one electron to become an ion. Atoms of which element have the same
number of electrons as a cesium ion?
10. Consider your answers to questions 6-9. What do all of the atoms you named have in common?
Model 2
(Reference: Modern Chemistry pg. 159-160)
As you know, all of the noble gases are very stable. Ions form in such a way so that the ion will have the
same number of electrons as a noble gas. Take oxygen, for example. Oxygen has 8 electrons. To become
like a noble gas it could either gain two to become like neon or it could lose six to become like helium. As
a general rule, atoms will gain or lose the fewest number of electrons possible.
A cation is a positively charged ion. It has lost electrons, so it has more positive protons than negative
electrons. An anion is a negatively charged ion. It has gained electrons, so it has more negative electrons
than positive protons. (Memory hint: A “cat”ion is “paws”itively charged. An “an”ion is “a n”egative ion.)
Key Questions
11. What does an oxygen atom do when becoming an ion? (Does it gain or lose electrons and how
many?)
12. An oxygen atom has an overall neutral charge because it has an equal number of protons and
electrons. What is the overall charge on an oxygen ion?
13. Is an oxygen ion a cation or an anion?
14. Consider an aluminum atom.
a) To become like argon, would aluminum have to gain or lose electrons? How many?
b) To become like neon, would aluminum have to gain or lose electrons? How many?
c) Considering your answers to parts a and b, what does an aluminum atom do to become an ion?
d) What is the charge on an aluminum ion?
e) Is an aluminum ion a cation or an anion?
15. If the following atoms become ions, what will their charges be? Will they be cations or anions?
a) Ca
b) Cl
c) N
d) K
e) S
f) B
g) P
Page | 2
POGIL: Period Trends – Ions and Ionic Radius
Model 3
(Reference: Modern Chemistry pg. 159-160)
When an atom becomes an ion, its radius changes because the number of electrons in its energy levels has
changed. The diagrams below show the relative sizes of atoms of two elements and the ions they form.
Atom A
Ion A
Atom B
Ion B
Key Questions
16. Are cations larger or smaller than their atom?
17. Are anions larger or smaller than their atom?
18. Draw Bohr diagrams for an atom of sodium and an ion of sodium.
19. Does the amount of positive charge in the nucleus change when sodium becomes an ion? Does the
amount of negative charge in the electron cloud change when sodium becomes an ion?
20. Using your Bohr diagram and answers to the two questions above, explain why sodium’s ionic radius is
smaller than its atomic radius.
21. Draw a Bohr diagram for an ion of lithium.
22. Using your Bohr diagrams for the lithium ion and the sodium ion, how does ionic radius change as you
go down a column on the periodic table? Explain this trend.
23. Draw Bohr diagrams for an atom of nitrogen and an ion of nitrogen.
24. Does the amount of positive charge in the nucleus change when nitrogen becomes an ion? Does the
amount of negative charge in the electron cloud change when nitrogen becomes an ion?
25. Using your Bohr diagram and answers to the two questions above, explain why nitrogen’s ionic radius
is larger than its atomic radius.
Page | 3
POGIL: Period Trends – Ions and Ionic Radius