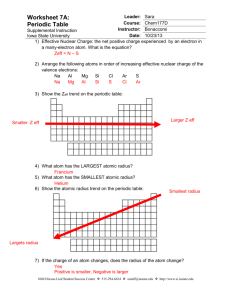
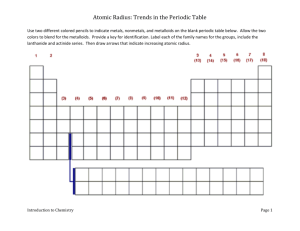
atomic radius ws - Copley
advertisement

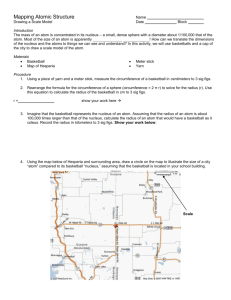
Name_______________________ Atomic Mapping The nucleus of an atom contains the vast majority of the mass of an atom, but only 1/100,000 of the volume. A bowling ball in front of the classroom is going to be used to measure represent the nucleus of the atom. How big would the electron cloud of this atom be? Procedure 1. Measure the circumference of the bowling ball. To do this, measure the area around the ball, using a flexible ruler in centimeters. Record your answer below 2. C= __68.5 cm_____ 3. Divide the circumference by 2 to get the radius of the bowling ball. Record your answer below. 4. R= ____________ 5. The radius of the atom is 100,000 times larger than the radius of the nucleus so multiply your radius by 100,000. Then convert your measurement from centimeters to meters, so divide by 100. 6. Record this distance below 7. R= _____________ 8. The map on the reverse has a scale in the upper left corner. It has a white bar with the number 3200 m. Measure the white bar in centimeters. Record its value below. 9. ________________ 10. To determine how big of a circle to draw. Multiply your values for #7 x #9 and divide by the 3200. This is the radius of your atom in centimeters on the map on the reverse. Record it below. 11. ________________ 12. Draw a circle on the map on the reverse side of this paper with a radius that you calculated in #11 using the star, the location of the high school, as the center. 13. This would be the size of an atom with a radius the size of this bowling ball. Map of Copley