periodictrends-ws-key1
advertisement
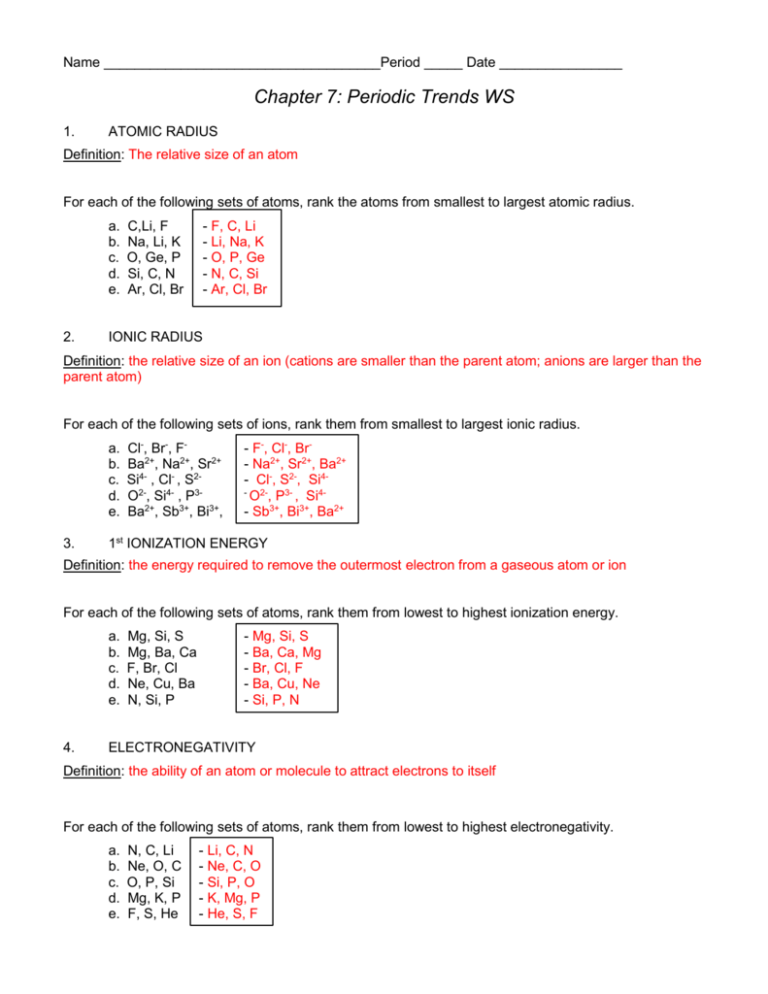
Name ____________________________________Period _____ Date ________________ Chapter 7: Periodic Trends WS 1. ATOMIC RADIUS Definition: The relative size of an atom For each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. a. b. c. d. e. 2. C,Li, F Na, Li, K O, Ge, P Si, C, N Ar, Cl, Br - F, C, Li - Li, Na, K - O, P, Ge - N, C, Si - Ar, Cl, Br IONIC RADIUS Definition: the relative size of an ion (cations are smaller than the parent atom; anions are larger than the parent atom) For each of the following sets of ions, rank them from smallest to largest ionic radius. a. b. c. d. e. 3. Cl-, Br-, FBa2+, Na2+, Sr2+ Si4- , Cl- , S2O2-, Si4- , P3Ba2+, Sb3+, Bi3+, - F-, Cl-, Br- Na2+, Sr2+, Ba2+ - Cl-, S2-, Si4O2-, P3- , Si4- Sb3+, Bi3+, Ba2+ 1st IONIZATION ENERGY Definition: the energy required to remove the outermost electron from a gaseous atom or ion For each of the following sets of atoms, rank them from lowest to highest ionization energy. a. b. c. d. e. 4. Mg, Si, S Mg, Ba, Ca F, Br, Cl Ne, Cu, Ba N, Si, P - Mg, Si, S - Ba, Ca, Mg - Br, Cl, F - Ba, Cu, Ne - Si, P, N ELECTRONEGATIVITY Definition: the ability of an atom or molecule to attract electrons to itself For each of the following sets of atoms, rank them from lowest to highest electronegativity. a. b. c. d. e. N, C, Li Ne, O, C O, P, Si Mg, K, P F, S, He - Li, C, N - Ne, C, O - Si, P, O - K, Mg, P - He, S, F More Exercises: 1. In each of the following sets, circle the element with the highest first ionization energy: (a) Li, K, Cs (b) S, Cl, Ar, (c) Br, I, Te (d) Cl , Na, Na+ ______________________________________________________________________________ 2. In each of the following sets, circle the species with the largest atomic radius: (a) Sr, Rb, Ba (b) O2-, S, S2(c) Cu, Ni, Co (d) K2+ ,Ca2+, Ga2+, (e) P, N, As ______________________________________________________________________________ 3. Circle the best choice in each list: (a) highest first ionization energy: N, B, Li (b) highest electronegativity: Te, Se, S (c) smallest atom: K, Fr, Be (d) highest (most negative) electron affinity: Se, Cl, Sb (e) lowest first ionization energy: Ca, Ga, K (f) highest second ionization energy: Na, Mg, Al (g) lowest second ionization energy: Ar, K, Ca _____________________________________________________________________________ 4. Define the term isoelectronic. Give an example of an isoelectric series that contains 5 elements or ions. The term isoelectronic means having the same number of electrons. An example of an isoelectronic series would be P3-, S2-, Cl-, Ar, K+ (all have 18 electrons). 5. What is the difference between electronegativity and electron affinity? Electronegativity is the ability for an atom/ion/molecule to attract electrons to itself whereas electron affinity is the energy change associated with the gain of an electron. For nonmetals, the gain of an electron is favorable and thus results in the atom releasing energy (exothermic) leaving the atom at a lower overall energy thus making it more stable. 6. Write the noble gas electron configuration of the following. (a) Ba2+ [Xe] [Kr]5s24d105p6 (b) Al3+ [Ne] [He]2s22p6 (c) Br - [Kr] [Ar]4s23d104p6 (d) Mg+ looks like Na [Ne]3s1 (e) S2- [Ar] [Ne]3s23p6 (f) N2- looks like F [He]2s22p5