S2 HW 5 Writing formula
advertisement
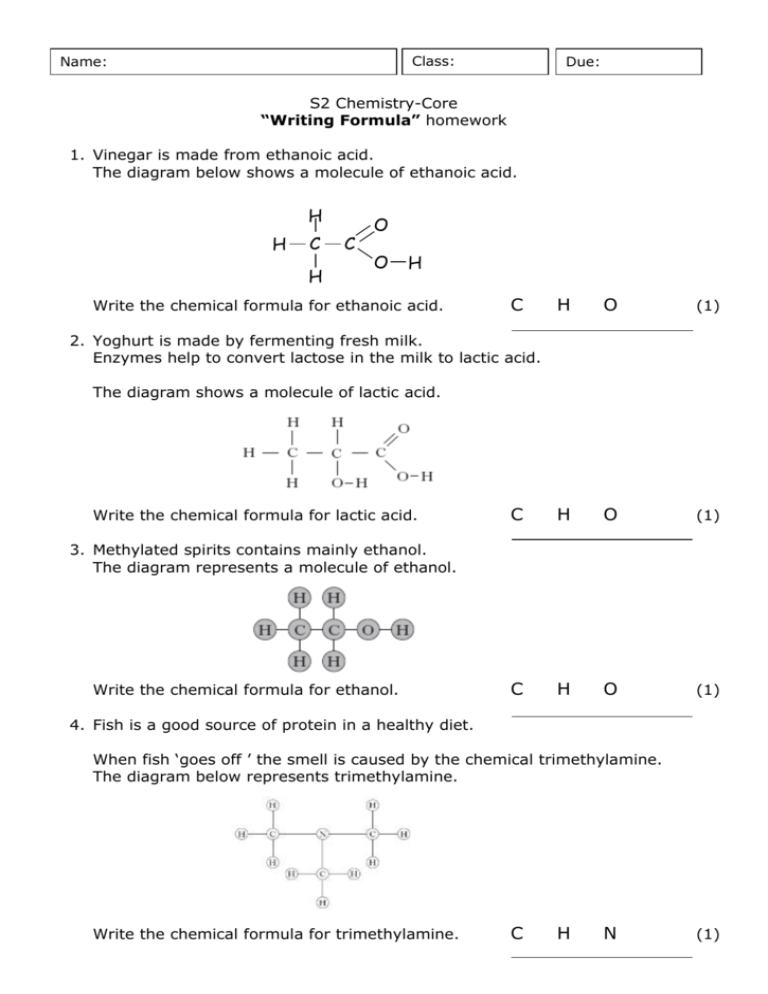
Class: Name: Due: S2 Chemistry-Core “Writing Formula” homework 1. Vinegar is made from ethanoic acid. The diagram below shows a molecule of ethanoic acid. H H C H C O O H Write the chemical formula for ethanoic acid. C H O (1) C H O (1) C H O (1) 2. Yoghurt is made by fermenting fresh milk. Enzymes help to convert lactose in the milk to lactic acid. The diagram shows a molecule of lactic acid. Write the chemical formula for lactic acid. 3. Methylated spirits contains mainly ethanol. The diagram represents a molecule of ethanol. Write the chemical formula for ethanol. 4. Fish is a good source of protein in a healthy diet. When fish ‘goes off ’ the smell is caused by the chemical trimethylamine. The diagram below represents trimethylamine. Write the chemical formula for trimethylamine. C H N (1) 5. Hydrazine is a fuel used in rockets. The diagram represents a molecule of hydrazine. (a) Write the chemical formula for hydrazine. N H (1) Hydrazine is unstable and can break down to produce ammonia, nitrogen and hydrogen. (b) Write a word equation for this reaction. + (1) + 6. Cubane is a compound of carbon and hydrogen that can be used as an explosive. The table shows the melting point of cubane and other carbon compounds. (a) Complete the table by adding the molecular formula of cubane. (1) (b) What is unusual about the melting point of cubane, when it is compared to the other melting points in the table? (1) 7. Ozone forms naturally in the upper atmosphere from oxygen molecules. It protects the Earth from harmful UV radiation and is known as the ozone layer. The diagram shows a molecule of ozone. (a) Write the chemical formula for ozone. O (1) Sunscreens protect skin by absorbing UV radiation. There are three types of UV radiation: UVA, UVB and UVC. The graphs show how three sunscreens absorb UV radiation. UVB radiation causes the skin to go red. (b) Which of these sunscreens would be best at stopping the skin from going red? Sunscreen (1) Score:













