Electronegativity and Polar Covalent Bonds
advertisement
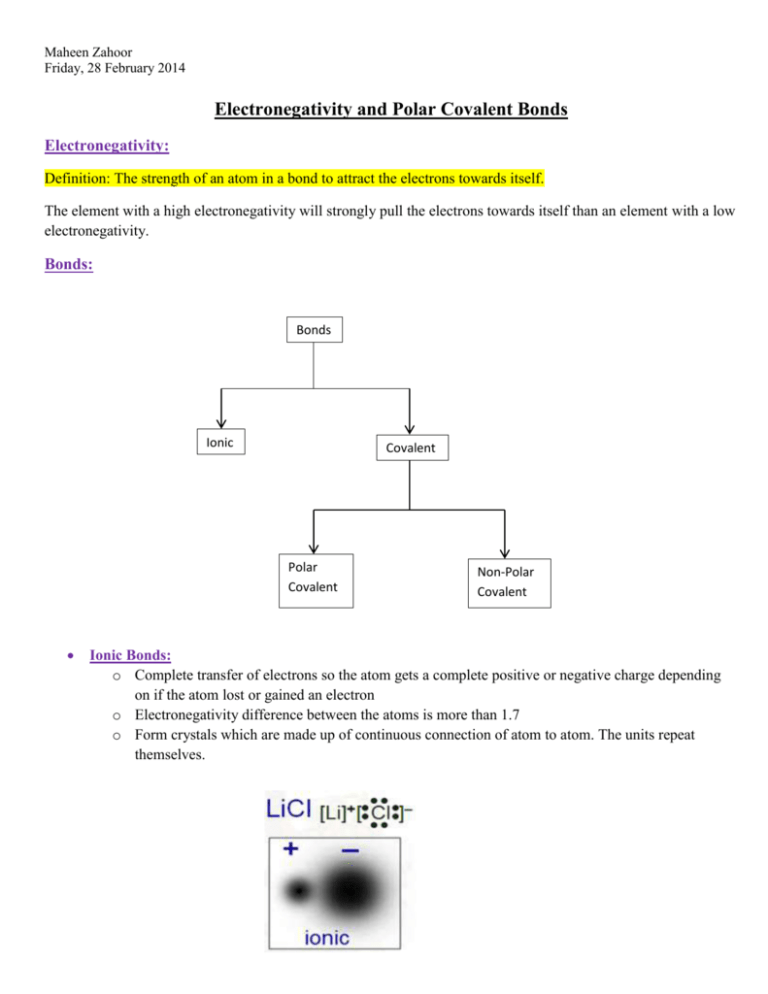
Maheen Zahoor Friday, 28 February 2014 Electronegativity and Polar Covalent Bonds Electronegativity: Definition: The strength of an atom in a bond to attract the electrons towards itself. The element with a high electronegativity will strongly pull the electrons towards itself than an element with a low electronegativity. Bonds: Bonds Ionic Covalent Polar Covalent Non-Polar Covalent Ionic Bonds: o Complete transfer of electrons so the atom gets a complete positive or negative charge depending on if the atom lost or gained an electron o Electronegativity difference between the atoms is more than 1.7 o Form crystals which are made up of continuous connection of atom to atom. The units repeat themselves. Maheen Zahoor Friday, 28 February 2014 Covalent Bonds: o Formation of individual molecules by sharing of electrons Non-Polar Covalent Bonds: o Equal sharing of electrons around the atoms that share those electrons o Electronegativity difference between the atoms is below 0.4 which means that both the atoms attract the electrons equally. Therefore, there are no partial charges. Polar Covalent Bonds: o Electronegativity difference between the atoms of the molecule is between 0.4 and 1.7 o The one that has a higher electronegativity among the two atoms attracts the electrons the most in the molecule. Therefore, the shared electrons spend most of their time around the atom that is more electronegative. This is unequal sharing because the atom with less electronegativity barely gets the electrons. o The “almost” absence of electrons on the less electronegative atom gives it a partial positive charge and the other atom a partial negative charge. It is partial because there is not a complete gain and loss. o The Greek symbol indicates “partial charge”. Maheen Zahoor Friday, 28 February 2014 Bonding Continuum: (The chart behind our periodic tables) o It is a GUIDELINE to determine if the bond is ionic, non-polar covalent or polar covalent. 0 0.4 Covalent 1.7 Polar Covalent 3.2 Ionic 100% Covalent 100% Ionic Increase in electronegativity. As the electronegativity increases the bonds go more towards being ionic because we are slowly moving towards a complete transfer of the electrons. To be completely sure if a substance is covalently bonded or if it is ionic, experiments need to be performed to gather data that will help us identify the bond. To understand better, watch this three minute video and learn an hour of stuff in 3 MINUTES WHILE SINGING! Catchy tune will help you remember. http://www.youtube.com/watch?v=oNBzyM6TcK8 Homework: Pg 73 # 1 – 6 Maheen Zahoor Friday, 28 February 2014 Pg 107 # 1 Pg 108 # 1, 4 – 6 Textbook Reading on Topic: Pg # 70 – 73, 102 – 108