DILI_implementation_030513_Mfld
advertisement

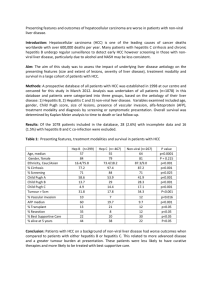
1 Marshfield Clinic – DILI EHR algorithm implementation (March 5, 2013) Administration of drug 1) Is the drug administered between D3 and D1? (anchor is D3, date of acute liver injury diagnosis) D2 Administration of drug Laboratory measurements 30 days D0 2) Are laboratory values normal between D0 and D2? (anchor is D2, date of drug administration) Acute Liver Injury Diagnosis D3 90 days D1 180 days D4 Laboratory measurements 3) Do laboratory values reach the threshold for DILI between D2 and D4? (anchor is D2, date of drug administration) Baseline population Biobank population for eMERGE (N=4762). Age >=18 years old. Acute liver injury diagnosis Structured data (ICD-9 codes) Date of instance of acute liver injury code, becomes D3. Acute liver injury related diagnoses and procedures Disorders of bilirubin excretion Acute and subacute necrosis of the liver Hepatic coma (hepatorenal syndrome) Hepatorenal syndrome Other disorders of the liver, including chemical or drug induced Other specified disorders of biliary tract Jaundice, unspecified, not of newborn Hepatomegaly Nonspecific elevation of transaminase or lactic dehydrogenase levels Abnormal liver function test results ICD-9 codes 277.4 570* 572.2 572.4* 573* 576.8 782.4 789.1* 790.4* 794.8* Administration of drugs Structured data (MedsManager) and NLP (FreePharma Notes). Date of a new medication order within 90 days prior to D3, becomes D1. Limited to Drug Induced Iiver Injury (DILIN) study protocol medication preparations: ciprofloxacin, clinafloxacin, levofloxacin, moxifloxacin, norfloxacin, ofloxacin, sparfloxacin,trovafloxacin, isoniazid, phenytoin, amoxicillin, valproic acid, nitrofurantoin, trimethoprim,minocycline. Laboratory measurements Upper limit of normal (ULN) is International Serious Events (iSAEC) study protocol specified. o ALP (Alkaline Phosphatase) ULN: 120 U/L (international units/liter). o ALT (Alanine Aminotransferase) ULN: 45 U/L (international units/liter). o Bilirubin (Indirect or Unconjugated) ULN: 40 umol/L (2.34 mg/dL). 2 Threshold laboratory values for DILI o ALP ≥ 2x ULN. o ALT ≥ 5x ULN. o ALT ≥ 3x ULN AND Bilirubin ≥ 2x ULN. All laboratory values should be below thresholds for DILI (normal) between D0 and D1. (ALP < 2x ULN, and ALT < 3x ULN, and Bilirubin < 2x ULN). Date laboratory values reach threshold for DILI between D2 and D4, becomes D5 (not shown). Excluded diagnoses Structured data only (ICD-9 codes) Chronic liver disease; Sclerosing cholangitis; organ transplantation or liver operation; alcohol abuse/liver damage/toxic effects; viral hepatitis. Chronic liver disease diagnosis description Alcoholic fatty liver Chronic hepatitis Chronic hepatitis, unspecified Chronic persistent hepatitis Other chronic hepatitis Cirrhosis of liver without mention of alcohol Biliary cirrhosis Other chronic nonalcoholic liver disease Autoimmune hepatitis Unspecified Chronic Liver Disease without Mention of Alcohol ICD-9 code 571.0 571.4 571.40 571.41 571.49 571.5 571.6 571.8 571.42 571.9 Sclerosing cholangitis description Cholangitis ICD-9 code 576.1 Organ transplantation and liver therapeutic operation descriptions ICD-9 code 996.81 996.82 996.83 996.84 996.85 996.86 996.89 998.87 998.88 996.80 V42.0 V42.1 V42.2 V42.3 V42.4 V42.5 V42.6 V42.7 V42.89 V42.81 Compl kidney transplant Compl liver transplant Compl heart transplant Compl lung transplant Complications of bone marrow transplant Compl pancreas transplnt Complications of other transplanted organ Complication of intestine transplantation Complications of transplanted organ Complications of transplanted organ Kidney replaced by transplant Heart replaced by transplant Heart valve replaced by transplant Skin replaced by transplant Bone replaced by transplant Cornea replaced by transplant Lung replaced by transplant Liver replaced by transplant Other specified organ or tissue replaced by transplant Bone marrow replaced by transplant 3 Peripheral stem cells replaced by transplant Pancreas replaced by transplant Intestines replaced by transplantation Unspecified organ or tissue replaced by transplant Allogeneic bone marrow transplant with purging Allogeneic bone marrow transplant without purging Cord blood stem cell transplant Autologous hematopoietic stem cell transplant with purging Allogeneic hematopoietic stem cell transplant Autologous bone marrow transplant with purging Autologous bone marrow transplant without purging Autologous hematopoietic stem cell transplant without purging Allogeneic hematopoietic stem cell transplant without purging Bone marrow transplant Unilat lung transplant Bilateral lung transplantation Lung transplantation Reimplantation of pancreatic tissue Homotransplant of pancreas Heterotransplant of pancreas Autotransplantation of cells of islets of langerhans Allotransplantation of cells of islets of langerhans Transplantation of cells of islets of langerhans Operations on pancreas: pancreatic transplant Hepatotomy Marsupialization of lesion of liver Partial hepatectomy Lobectomy of liver Total hepatectomy Auxiliary liver transplant Other transplant of liver Closure of laceration of liver Other repair of liver Percutaneous aspiration of liver Extracorporeal hepatic assistance Localized perfusion of liver Other injection of therapeutic substance into liver Unlisted proc 50.99 Open ablation of liver lesion or tissue Percutaneous ablation of liver lesion or tissue Laparoscopic ablation of liver lesion or tissue Other and unspecified ablation of liver lesion or tissue Other destruction of lesion of liver ICD-9 code V42.82 V42.83 V42.84 V42.9 41.02 41.03 41.06 41.07 41.08 41.09 41.01 41.04 41.05 41.00 33.51 33.52 33.50 52.81 52.82 52.83 52.84 52.85 52.86 52.80 50.0 50.21 50.22 50.3 50.4 50.51 50.59 50.61 50.69 50.91 50.92 50.93 50.94 50.99 50.23 50.24 50.25 50.26 50.29 Alcohol Abuse, Liver Damage, and Toxic Effects Descriptions Alcohol Abuse, Unspecified Drinking Behavior Alcohol Abuse, Continuous Drinking Behavior Alcohol Abuse, Episodic Drinking Behavior Alcohol Abuse, In Remission Alcoholic fatty liver Acute alcoholic hepatitis ICD-9 code 305.00 305.01 305.02 305.03 571.0 571.1 Organ transplantation and liver therapeutic operation descriptions 4 Alcohol Abuse, Liver Damage, and Toxic Effects Descriptions Alcoholic cirrhosis of liver Alcoholic liver damage Toxic effect of ethyl alcohol Toxic effect of methyl alcohol Toxic effect of isopropyl alcohol Toxic effect fusel oil Toxic effect of other specified alcohols Toxic effect of unspecified alcohol ICD-9 code 571.2 571.3 980.0 980.1 980.2 980.3 980.8 980.9 Viral Hepatitis Descriptions Unspecified Viral Hepatitis Without Mention Of Hepatic Coma Unspecified Viral Hepatitis With Hepatic Coma Other Specified Viral Hepatitis With Hepatic Coma Other Specified Viral Hepatitis Without Mention Of Hepatic Coma Viral Hepatitis A Without Mention Of Hepatic Coma Viral Hepatitis A With Hepatic Coma Viral Hepatitis B Without Mention Of Hepatic Coma, Acute Or Unspecified Viral Hepatitis B With Hepatic Coma, Acute Or Unspecified Viral Hepatitis B With Hepatic Coma, Acute Or Unspecified, With Hepatitis Delta Viral Hepatitis B, Acute Or Unspecified, With Hepatitis Delta Acute Hepatitis C With Hepatic Coma Hepatitis Delta Without Mention Of Active Hepatitis B Disease With Hepatic Coma Hepatitis E With Hepatic Coma Unspecified Viral Hepatitis C Without Hepatic Coma Hepatitis In Viral Diseases Classified Elsewhere Carrier Or Suspected Carrier Of Viral Hepatitis Chronic Viral Hepatitis B Without Mention Of Hepatic Coma Or Hepatitis Delta Viral Hepatitis B With Hepatic Coma, Chronic, Without Mention Of Hepatitis Delta Viral Hepatitis B With Hepatic Coma, Chronic, With Hepatitis Delta Viral Hepatitis B Without Mention Of Hepatic Coma, Chronic, With Hepatitis Delta Other Icd9 Viral Hepatitis Carrier Unspecified Viral Hepatitis C With Hepatic Coma ICD-9 code 070.9 070.6 070.49 070.59 070.1 070.0 070.30 070.20 070.21 070.31 070.41 070.42 070.43 070.70 573.1 V02.60 070.32 070.22 070.23 070.33 V02.69 070.71 Algorithm results 22 DILI cases as defined by algorithm 1657 controls (No acute liver injury diagnoses, has exposure to drug, and no exclusion diagnoses). Validation steps Manual chart review of 10 randomly selected DILI cases (10 of 22 above). 1 clinical reviewer (senior research coordinator) Evaluation of algorithm-selected cases Cases Total algorithm selected DILI cases True positive cases (TPs) False positive cases (FPs) Positive predictive value: TP/(TP+FP) Counts 10 1 9 10%