Moe`s Mysterious Metaphoric Meanderings Time to get sciency
Moe’s Mysterious Metaphoric Meanderings
TIME TO GET SCIENCY: INFLUENCE OF POLARITY ON SHAPE
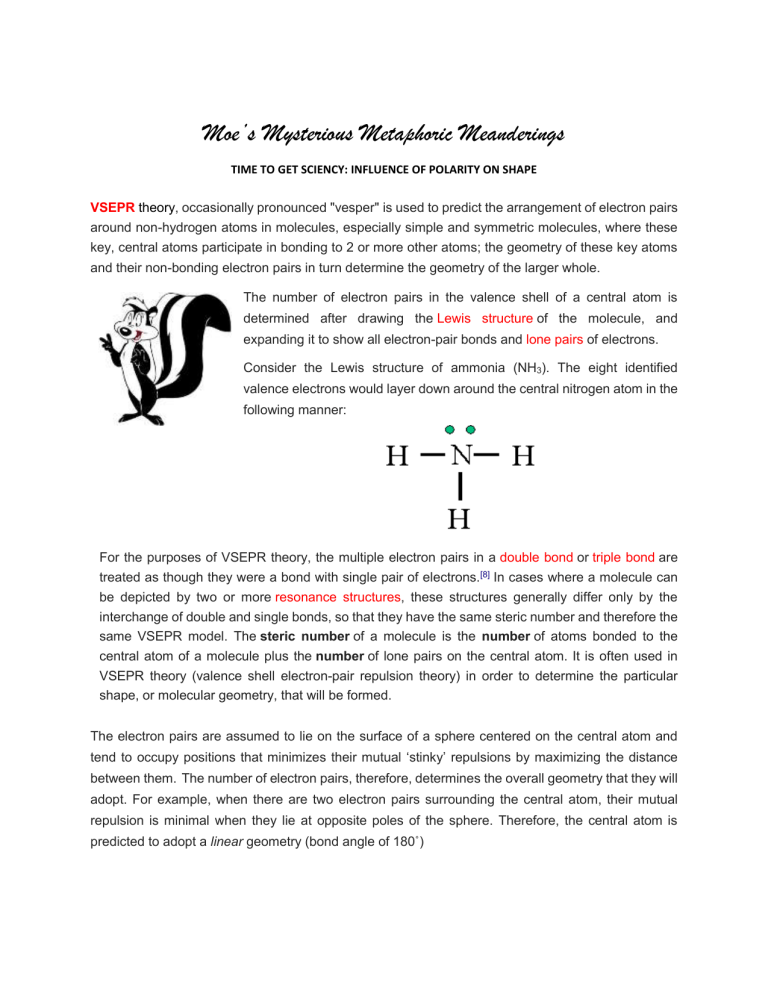
VSEPR theory , occasionally pronounced "vesper" is used to predict the arrangement of electron pairs around non-hydrogen atoms in molecules, especially simple and symmetric molecules, where these key, central atoms participate in bonding to 2 or more other atoms; the geometry of these key atoms and their non-bonding electron pairs in turn determine the geometry of the larger whole.
The number of electron pairs in the valence shell of a central atom is determined after drawing the Lewis structure of the molecule, and expanding it to show all electron-pair bonds and lone pairs of electrons.
Consider the Lewis structure of ammonia (NH
3
). The eight identified valence electrons would layer down around the central nitrogen atom in the following manner:
For the purposes of VSEPR theory, the multiple electron pairs in a double bond or triple bond are treated as though they were a bond with single pair of electrons.
[8] In cases where a molecule can be depicted by two or more resonance structures , these structures generally differ only by the interchange of double and single bonds, so that they have the same steric number and therefore the same VSEPR model. The steric number of a molecule is the number of atoms bonded to the central atom of a molecule plus the number of lone pairs on the central atom. It is often used in
VSEPR theory (valence shell electron-pair repulsion theory) in order to determine the particular shape, or molecular geometry, that will be formed.
The electron pairs are assumed to lie on the surface of a sphere centered on the central atom and tend to occupy positions that minimizes their mutual ‘stinky’ repulsions by maximizing the distance between them.
The number of electron pairs, therefore, determines the overall geometry that they will adopt. For example, when there are two electron pairs surrounding the central atom, their mutual repulsion is minimal when they lie at opposite poles of the sphere. Therefore, the central atom is predicted to adopt a linear geometry (bond angle of 180˚)
If there are 3 electron pairs surrounding the central atom, their repulsion is minimized by placing them at the vertices of an equilateral triangle centered on the atom. Therefore, the predicted geometry is trigonal . Likewise, for 4 electron pairs, the optimal arrangement is tetrahedra l .
This overall geometry is further refined by distinguishing between bonding and nonbonding electron pairs. VSEPR theory views repulsion by the lone pair to be greater than the repulsion by a bonding pair. As such, when a molecule has 2 interactions with different degrees of repulsion, VSEPR theory offers the structure where lone pairs occupy positions that allow them to experience less repulsion.
Lone pair-lone pair (lp-lp) repulsions are considered stronger than lone pair-bonding pair (lp-bp) repulsions, which in turn are considered stronger than bonding pair-bonding pair (bp-bp) repulsions, distinctions that then guide decisions about overall geometry when 2 or more non-equivalent positions are possible.
For instance, when 2 bonding pairs and3 lone pairs of electrons surround a central atom, a linear molecular geometry is specified.
In this geometry, the 2 collinear "axial" positions lie 180° apart from one another, and 90° from each of 3 adjacent "equatorial" positions; these 3 equatorial positions lie 120° apart from each other and experience only two closer proximity 90° neighbors (the axial positions). The axial positions therefore
experience more repulsion than the equatorial positions; hence, when there are lone pairs, they tend to occupy equatorial positions.
Alternatively, the difference between lone pairs and bonding pairs may also be used to rationalize deviations from idealized geometries. For example, the sulfur dioxide molecule (SO
2
) with one lone pair and three bond pairs (one single bond and one double bond):
The central pairs are spread so as to point roughly towards bent or angular configuration with a bond angle of less than the expected 120 had there been a third oxygen instead of a lone pair of electrons.
The lone pair of electrons (whose density or probability envelopes lie closer to the oxygen nucleus) exert a greater mutual repulsion than the two bond pairs.
INFLUENCE OF SHAPE ON POLARITY
When the atoms in a covalent bond have different electronegativities, the electrons are shared unequally and the bond is polar. Depending on the molecular shape, polar bonds can give rise to a polar molecule, one with an overall uneven distribution of electron charge. We can see this charge imbalance when the molecules are placed in an electric field. Polarity is a key feature of a molecule because it can influence physical, chemical, and even biological properties.
For a diatomic molecule, a polar bond must lead to a polar molecule. Consider hydrogen fluoride, shown here using a space-filling format where the intensity of the colors describe how the electrons distribute themselves between the hydrogen and fluorine nuclei..
Note the positive and negative delta symbols indicating that the molecule is somewhat polar. If red indicates high electron density and green indicates low, you can see that the F end of the molecule is much more negative than the H end, and thus HF is highly polar. Between two electric plates with
the field off, the molecules lie every which way. With the field on, however, they become oriented with their negative ends facing the positive plate and their positive ends facing the negative plate.
If a molecule has more than two atoms, its shape can affect the polarity in a crucial way. For example, in carbon dioxide, since oxygen is more electronegative than carbon, each bond is highly polar. But the linear molecular shape makes the bond polarities cancel each other, so the CO2 molecule is nonpolar.
The situation is very different for water. As in CO2, the highly electronegative oxygen pulls electron density toward itself, so each bond is polar. But the V-shape of the molecule allows the bond polarities to reinforce each other, so water is highly polar; the two lone pair of electrons see to that. With the field off, the molecules are oriented randomly, but with it on, their poles become oriented toward the oppositely charged plates.
Consider these examples of shape determining polarity. The case of boron trifluoride (BF
3
) is similar to that of CO2. Because the three highly polar bonds point to the corners of an equilateral triangle, the bond polarities cancel each other, and BF3 is nonpolar
Note that Boron does not require a completed octet as part of its bonding criterion. Six electrons do quite well. Boron is an exception to the octet rule .
The covalently bonded compound, ammonia (NH
3
), provides us with an alternative situation. Unlike
Boron, nitrogen’s stability depended upon a completed octet.
Like BF3, ammonia (NH
3
) has four atoms (one nitrogen and three hydrogens) and three polar bonds, but its trigonal pyramidal shape results from the bond polarities reinforcing each other. Thus, ammonia is highly polar for the same reason that water is.
Sometimes two molecules have similar overall shapes, but their slightly different compositions lead to very different polarities. Carbon tetrachloride, for example, has four polar carbon-chlorine bonds that point to the corners of a tetrahedron, so they cancel each other and give a nonpolar molecule.
Substituting a hydrogen for one of the chlorines gives chloroform, another tetrahedral molecule, but now the polar bonds reinforce each other. As a result, chloroform is highly polar.
Revisiting Stinky Moe and his friends
Remember when we positioned clay spheres over a central larger sphere in order to minimize the effects of the stinky skunks? If you were to go back to your original models and determined the angles of separation between the skunks, what would you get?
Once you’ve opened up your treasure chests, examine the three dimensional models you previously constructed, take photographs or diagram the models for your records. Use a protractor to determine the angles of separation between the skunks and record your observations on the following table.
1
Angles Between Skunks
2.
Angles Between Skunks
3
Angles Between Skunks
4
Angles Between Skunks
5
Angles Between Skunks
6
Angles Between Skunks
Once you’ve determined the angles in your original models, establish whether your initial ideas were correct based upon what you know about VSEPR. You will need to collect the following information:
1. Electron Geometry (name of geometry and angles between electron pairs)
2. Molecular Geometry (symbol (eg AX
2
E
1
), molecular shape and bond angles between terminal atoms)
3. Construct the molecule using molecular models in the lab. Photograph or diagram the completed model for your records
4. Predict whether the molecule is polar or nonpolar.
5. Provide a real-world example of the model (eg. AX
2
E
1
describes sulfur dioxide (SO
2
))
Welcome to the Real World:
Number of
Electron pairs
Electron Pair
Geometry
Molecular
Geometry
Bond angles Polarity Examples Stinky
Scenario
5 stinky skunks
4 stinky skunks,1
Moe
4 stinky skunks
3 stinky skunks,1
Moe
3 stinky skunks
2 stinky skunks, 1
Moe
Putting It Altogether
We are at the point where we ought to be able to look at the formula for, say, chlorine trifluoride (ClF
3
) and determine both the molecular geometry and the polarity for the molecule.
First, a quick review of Lewis structures. How about determining the outline of how to determine the
"best" Lewis structure for an example, the nitrate polyatomic ion (NO
3
):
1. Determine the total number of valence electrons in a molecule, or in this case a polyatomic ion
(NO
3
)
2. Draw a skeleton for the molecule which connects all atoms using only single bonds. In simple molecules, the atom with the most available sites for bondng is usually placed central. The number of bonding sites are determined by considering the number of valence electrons and the ability of an atom to produce an octet. As you become better, you will be able to recognize that certain groups of atoms prefer to bond together in a certain way. It more sense for nitrogen to be the central atom.
3. Of the 24 valence electrons in NO
3
, 6 were required to bond the central atom to the terminal atoms. Consider the remaining 18 electrons and place them so as to fill the octets of as many atoms as possible.
4. Are the octets of all the atoms filled? If not, then fill the remaining octets by making multiple bonds.
Finding a Model that works