Shapes of Molecules
advertisement
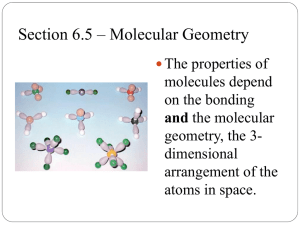
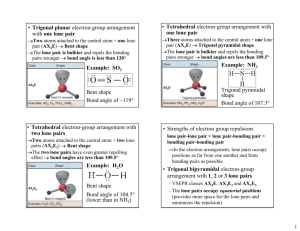
Shapes of Molecules Learning objectives • Explain the shape of simple molecules based on repulsion of electron pairs around a central atom. • Understand that lone pairs repel more than bonded pairs • Explain the shapes and bond angles in trigonal planar, tetrahedral, octahedral, pyramidal, nonlinear and linear. Covalent bonds Unlike Group 1 and group 7 elements, most elements need to gain, lose or share more than one electron Draw a dot cross diagram for oxygen What holds covalent bonds together? What force exists between two atoms? The Electrostatic force Draw a diagram to show these forces The atomic separation of particles in a nucleus is determined by the balance of these forces Lewis Diagrams A Lewis diagram consists of the element symbol surrounded by "dots" to represent the number of electrons in the outer energy level Draw Lewis diagrams for every element in the second period Shapes of molecules Electrons go in pairs (the pairs are in the same orbital). The electrons in the pairs aren’t repelling each other because of their spin. The shape of a molecule can be predicted based on the number and arrangement of electron pairs around a central atom Each electron pair will repel another electron as far away as it can This is sometimes known as VSEPR (Valency shell electron pair Repulsion) theory VSEPR Principle: Electron pairs around a central atom arrange themselves so that they can be as far apart as possible from each other. It is important to remember that Atoms are three dimensional objects In the diagrams the central atom is denoted by X and attached surrounding bonded atoms by Q. The bond angle is therefore based on angle between the atoms Q-X-Q. Therefore the total bond angle for a molecule in 2 Dimensions will be 360˚ Linear Molecules In a linear molecule, the atoms lie in a straight line. The bond angle formed is therefore 180°. Trigonal Planar molecule All four atoms lie on the same plane and the bond angles are all 120° Tetrahedral Molecules A tetrahedral molecule is a molecule which has four atoms extending out from a central atom. The four atoms are located at the vertices of a tetrahedron. All bond angles are 109.5°. Drawing molecules A simple straight line represents a bond lying approximately in the surface plane. A wedge shaped bond is directed in front of this plane (thick end toward the viewer), and a hatched bond is directed in back of the plane (away from the viewer), You may see a dashed bond used in place of A hatched bond Octagonal molecule It has six vertices with atoms all extending from a central atom. All bond angles are 90° Lone pairs What is a lone pair? Lone pairs occur in elements from group 5, 6 and 7 Lone pair How many lone pairs does Oxygen have? Lone pairs Lone pairs affect the shape of the molecule Lone pairs Any lone pairs of non-bonding electrons on the central atom X, are closer to X than bond pairs because there is no Q atom attracting/sharing the lone pair electron charge. This will increase the repulsion between a lone pair of electrons on X and any other bonding/non-bonding on X Pyramidal molecule There is a lone pair on the phosphorus atom So there is more repulsion from this lone pair, this means that the angles between Chlorine atoms will be slightly smaller Lone pairs Bent molecules Water has two lone pairs This means the two hydrogen atoms are pulled even closer together