The Effect of pH on the Reaction between Pepsin and Egg White
advertisement

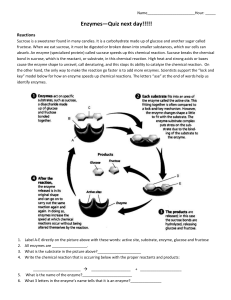
The Effect of pH on the Reaction between Pepsin and Egg White Introduction An enzyme is a biological catalyst (Purchon 2012). Its most elementary function is to speed up the rate of reaction (Enzymes (n.d.). Without the existence of enzymes within living organisms, the process of digestion would take weeks and the function of our muscles; nerves and bones will decrease in efficiency (Purchon 2012). Therefore the activities in living systems are controlled through and dependent on enzymes. Similar to other catalysts, enzymes can be reused multiple times, however their natural properties are easily taken. In order for enzymes to maintain their natural qualities, they must exist in body temperature and a specific pH (Purchon 2012). The reason why enzymes are so sensitive to heat and pH is because they are protein molecules (Purchon 2012). An enzyme will only function within its specific chemical reaction. How enzymes work is that they bind themselves to one reactant also known as a substrate (Enzymes (n.d.)), by doing this they are lowering the activation energy of the reaction that they are catalyzing, hence the speed of reaction is increased. There are many different types of enzymes within living organisms catalyzing specific chemical reactions. In this particular experiment, the enzyme pepsin was used. First recognized in 1836,pepsin is a digestive enzyme found in the gastric juices of the stomach of living organisms, mainly mammals (Pepsin 2012). It breaks down protein in foods such as meat, eggs, seeds and dairy products into peptides. Pepsin is formed by the release of pepsinogen in the stomach (Pepsin 2012). This is sparked by impulses from nerves and hormonal secretions of gastrin and secretin. The pepsinogen then is exposed to and mixes with the hydrochloric acid and rapidly unfolds and breaks, converting into pepsin(Pepsin 2012).To break proteins into basic amino acid molecules, the bonds that join the amino acids called the peptide bonds must be broken (Hendrickson 2010). The reaction that breaks these bonds is called hydrolysis, as a water molecule is required in order for the peptide bonds to split (Hendrickson 2011). During this reaction the peptide bond breaks, simultaneously the water molecule splits and a new bond is formed with one of the amino acids and the hydrogen atom from water, the other amino acid forms a bond with the other hydrogen atom and an oxygen atom present (Hendrickson 2011).Pepsin speeds up the hydrolysis of protein and makes this reaction more effective (Hendrickson 2011). Pepsin performs most proficiently at pH of 1.5 - 2.5, which is the normal acidity of gastric juices (Pepsin 2012). A pH any higher and the enzyme will cease to function properly (Hendrickson 2010). The enzyme is important for the absorption of nutrition from the protein consumed. The absence of pepsin would cause the digestive tract to move the food eaten through the stomach and intestine very quickly and would not give the body enough time to absorb the nutrition, as the rate of the hydrolysis reaction is too slow without pepsin (Hendrickson 2010). The small intestine may have absorbed some partially undigested (Carter 2012) or larger fragments of protein and so from the stomach, small amounts of pepsin are passed into the bloodstream where it breaks it all down. The aim of this experiment is to investigate the effect of different pH levels on the activity of pepsin on proteins. That protein that we used was egg white. The main objective was to model the activity of pepsin in the stomach by showing its actions on protein by simulating the effect with pepsin and egg whites. The experiment was carried out at a number of pH levels to show that pepsin (and all other enzymes) have a no ptimal pH range in which they perform most effectively, in order to model the effect the changing pH has on the activity of the enzyme. Enzyme has an optimal pH range and anything outside this range inhibits its activity (Hendrickson 2010), therefore it was hypothesized that the lower the pH, the faster the rate of enzyme reaction (pepsin) activity. Pepsin has an optimal range of pH 2(Pepsin 2012), which is highly acidic and is found in the stomach of the human body where it digests proteins. This means that any pH less acidic or more basic than this level will not allow the reaction to occur, as these are not the optimal conditions for the activity of the enzyme. Materials and Method 6 x Test tubes 30cm Pepsin 150ml egg white - suspension 1 x Test Tube Rack 1 x Stop Watch 10ml measuring cylinder 500ml beaker 25ml 1M HCl (pH 0) 25ml Citric buffer (pH 3) 25ml Citric buffer (pH 5) 25ml Phosphate buffer (pH 8) 25ml Phosphate buffer (pH 12) Graduated pipette Universal indicator strips Method: Six test tubes were collected, labeled one to six and placed on a test tube rack. In each of the labeled test tubes 5ml of egg - white suspension was placed into it using a clean graduated pipette. Test labeled one had 5ml of HCl (pH 0) added into it using a clean graduated pipette. 5mL of Citric buffer (pH 3) was added to test tube 2 and similarly test tube had 5ml of citric buffer (pH 5) added into it using clean graduated pipettes. 5ml of phosphate buffer (pH 8) and 5ml of phosphate buffer (pH 12) was added into test tubes4 and 5 respectively, using clean graduated pipettes. Test tube 6 was used as the control. The precise pH of each solution was compared by adding two drops of the sample, with a clean pipette, onto a universal indicator. This was done for each sample (Carter 2012).One centimeter of pepsin was added into each test tube 1 using a clean graduated pipette and the stopwatch was started simultaneously. The time in which it took the solution in test one to go clear was what was being measured. The results were recorded into the results table and the process of putting one centimeter of pepsin into test tube 1 and timing how long it takes for the solution to clear and recording the figures attained were repeated for test tubes 2-6 in order to ensure reliability of the results.(Steane R. (n.d.)).