SG- Unit 5 Gases
advertisement

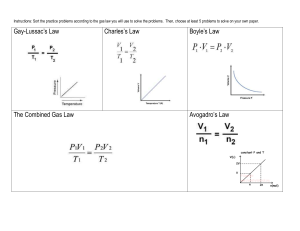
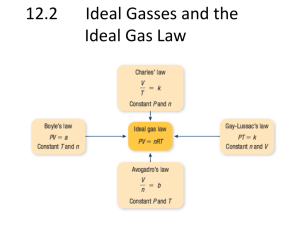
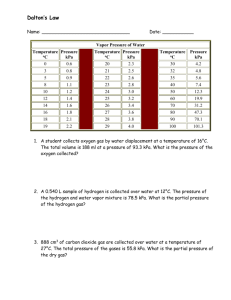
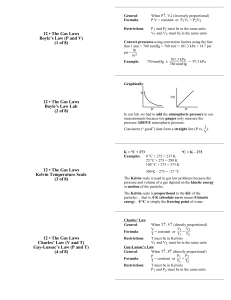
Study Guide Unit 5: Gas Laws Chapter 13.1 Use the textbook and notes to answer the following: 1. Kinetic Molecular Theory: 2. Elastic collision: 3. Diffusion: 4. Pressure: 1_________=760_______=760________=101.325___________=14.7__________ 5. Barometer: 6. A can filled with air is heated to force some of the air out of the can. When the can is capped and cooled it will crush because the pressure inside has ___________ due to ______________ molecules colliding against the surface. 7. STP stands for _________________________________________________________________________ At STP temperature can either be ______C or _______K. 8. What is the lowest possible temperature? _________________________ 9. Pg. 388 in your text states that ‘Gas particles exert pressure when _______________________________________________________________________________________.’ 10. Apply the concept in question 9 to tires of a car. How is the pressure in a tire created? 11. Diffusion occurs due to constant _____________ motion of gas molecules. 12. Kelvin = ______+ 0C Celcius = K - __________ Complete the following laws by writing the equation (know how to use them!) a. b. c. d. e. f. Boyle’s Law Charles’ Law Gay-Lussac’s Law Combined Gas Law Ideal Gas Law Dalton’s Law of Partial Pressure Solve and identify which law is used (yes that means you have to write which law it is.) 1. 300.0 mL of gas has a pressure 75.0 kPa. When the volume is decreased to 125.0 mL, what is its pressure? 2. 50.0 L of gas has a temperature of 75C. What is the temp in Celsius when the volume changes to 110 L? 3. What is the volume of a container that holds 48.0 g of helium at a pressure of 4.0 atm and temperature of 52C? 4. A gas occupies 325 L at 25C and 98.0 kPa. What is its volume at 70.0 kPa and 15C? 5. Krypton occupies 12.0L at a pressure of 156 kPa and a temperature of 245 Kelvin. Find how many moles of Krypton are present in the container. 6. A balloon contains 0.1 moles of oxygen and 0.4 moles of nitrogen. If the balloon is at standard temperature and pressure, what is the partial pressure of the nitrogen be in kPa? 7. What is the volume of a container that holds 2.5 moles of carbon dioxide at STP? (Hint: what are the standard temp and pressure that match the R value?)