HW 9 Key - EPIC Chemistry
advertisement
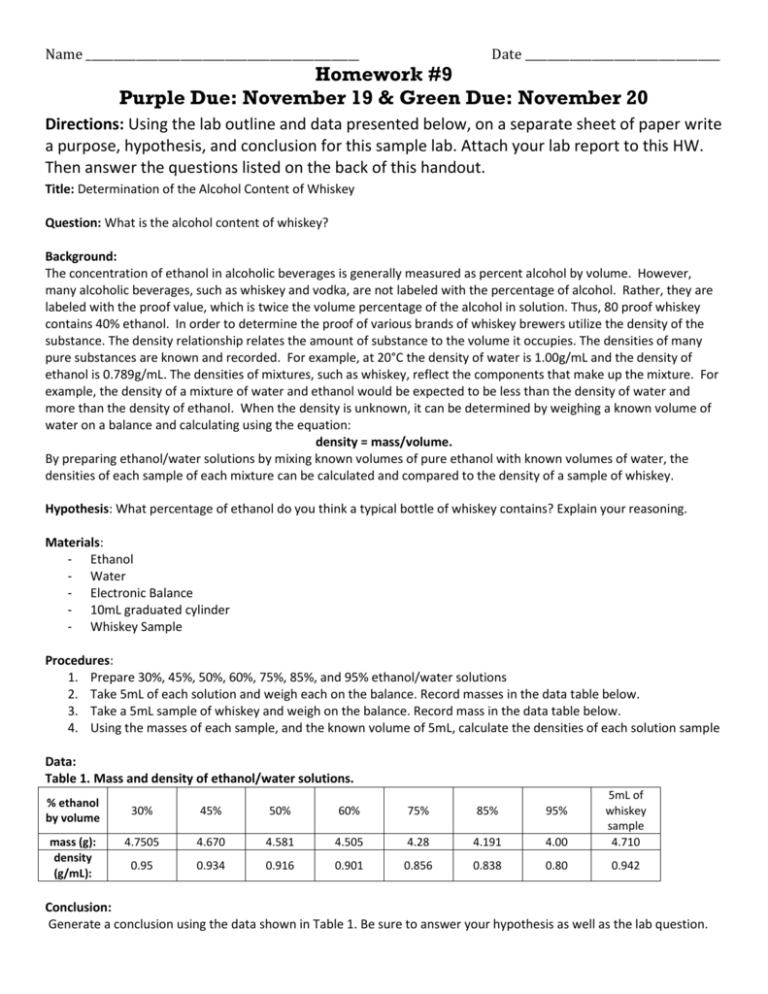
Name _________________________________________________ Date ___________________________________ Homework #9 Purple Due: November 19 & Green Due: November 20 Directions: Using the lab outline and data presented below, on a separate sheet of paper write a purpose, hypothesis, and conclusion for this sample lab. Attach your lab report to this HW. Then answer the questions listed on the back of this handout. Title: Determination of the Alcohol Content of Whiskey Question: What is the alcohol content of whiskey? Background: The concentration of ethanol in alcoholic beverages is generally measured as percent alcohol by volume. However, many alcoholic beverages, such as whiskey and vodka, are not labeled with the percentage of alcohol. Rather, they are labeled with the proof value, which is twice the volume percentage of the alcohol in solution. Thus, 80 proof whiskey contains 40% ethanol. In order to determine the proof of various brands of whiskey brewers utilize the density of the substance. The density relationship relates the amount of substance to the volume it occupies. The densities of many pure substances are known and recorded. For example, at 20°C the density of water is 1.00g/mL and the density of ethanol is 0.789g/mL. The densities of mixtures, such as whiskey, reflect the components that make up the mixture. For example, the density of a mixture of water and ethanol would be expected to be less than the density of water and more than the density of ethanol. When the density is unknown, it can be determined by weighing a known volume of water on a balance and calculating using the equation: density = mass/volume. By preparing ethanol/water solutions by mixing known volumes of pure ethanol with known volumes of water, the densities of each sample of each mixture can be calculated and compared to the density of a sample of whiskey. Hypothesis: What percentage of ethanol do you think a typical bottle of whiskey contains? Explain your reasoning. Materials: - Ethanol - Water - Electronic Balance - 10mL graduated cylinder - Whiskey Sample Procedures: 1. Prepare 30%, 45%, 50%, 60%, 75%, 85%, and 95% ethanol/water solutions 2. Take 5mL of each solution and weigh each on the balance. Record masses in the data table below. 3. Take a 5mL sample of whiskey and weigh on the balance. Record mass in the data table below. 4. Using the masses of each sample, and the known volume of 5mL, calculate the densities of each solution sample Data: Table 1. Mass and density of ethanol/water solutions. % ethanol by volume mass (g): density (g/mL): 30% 45% 50% 60% 75% 85% 95% 4.7505 4.670 4.581 4.505 4.28 4.191 4.00 5mL of whiskey sample 4.710 0.95 0.934 0.916 0.901 0.856 0.838 0.80 0.942 Conclusion: Generate a conclusion using the data shown in Table 1. Be sure to answer your hypothesis as well as the lab question. Directions: The following questions are based on the lab describe above, use the information above in order to answer the questions below. 1. Identify as many relationships as possible seen in Table 1 provided in the sample lab and record them in the space below. Be clear on what variables you are comparing. Hint: there are three variables shown in Table 1. % ethanol by volume and mass are inversely related % ethanol by volume and density are inversely related Mass and density are directly related 2. Which of the following generalizations is best supported by the data provided in Table 1? a. The tested sample of whiskey has a relative percent ethanol content of 40% b. The tested sample of whiskey has a relative percent ethanol content of 35% c. The tested sample of whiskey has a relative percent ethanol content of 85% d. The tested sample of whiskey has a relative percent ethanol content of 75% 3. A second brand of whiskey was tested in order to determine which of the two brands has the highest alcohol content by percentage. If the second brand of whiskey had a density around 0.85g/mL what would be the percentage ethanol content by volume? Use the data table provided in the sample lab to help you. a. 75% b. 85% c. 80% d. 30% 4. Which of the following conclusions is best supported by the data provided in the sample lab? a. As the mass of the solution samples increases, the percent ethanol content increases b. Solution samples with higher percent ethanol by volume have lower densities c. The greater the mass of a solution sample, the lower the density d. As the percent ethanol by volume content of the different solution samples increased, the densities increased








