Mitochodrial Isolation
advertisement

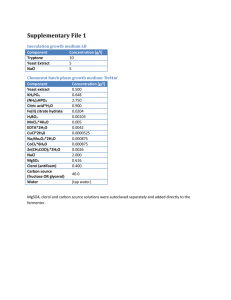
Standard Operating Procedure Title: Mitochondrial Isolation from Etiolated Corn Shoots Department: Agronomy Created by: Chris Hejlik Laboratory: Crop Production & Physiology Lab Suite Supervisor: Dr. Allen Knapp Lab Supervisor: Whitney Bouma Date Approved:__________________ Procedure Overview: The procedure provided is for the mitochondrial isolation of etiolated corn shoots after approximately seven days. Isolated mitochondria are to be used for gradient purification, integrity checks by ADP/O ratio measurements, as well as respiration measurements. MSDS Information: MSDS Sheets: Available for reference in laboratory 1522 Chemicals: Mannitol MOPS EGTA BSA Cysteine KOH Equipment and reagents necessary: Plant Material(s) 1) Etiolated Seedlings grown from the following lots a. M808 E-10 VT3 RRs seed lots, 108 days maturity i. Seed lot treated with active ingredient and fillers ii. Seed lot treated with fillers iii. Seed lot with no treatment b. M314 A-10 VT3 RR2 seed lots, 114 days maturity i. Seed lot treated with active ingredient and fillers ii. Seed lot treated with fillers iii. Seed lot with no treatment Equipment 1) Waring Blender 2) Multiple layers of cheesecloth Centrifuge, CP & P Lab a. Centrifuge tubes i. 503) Growth Chamber 4) Scissors or razor blade 5) Ruler 6) Various beakers a. 50b. 100c. 250-ml Reagents 1) Grinding Medium a. 350 mM Mannitol b. 30 mM MOPS c. 1 mM EGTA 2) 8 M KOH 3) Wash Medium a. 350 mM Mannitol b. 20 mM MOPS c. 1 mM EGTA 4) 0.84 g PVPP per trial 5) .1634 g cysteine per trial Procedure 1. Cut etiolated corn shoots into segments 0.5 - 1.0 cm long using a razor blade; material should include mesocotyls only 2. Grinding medium, cysteine, and PVPP added to Waring Blender. a. Grinding medium added in a 3.33:1 ratio to starting fresh weight 3. Lid of Waring Blender added, and homogenization started a. Homogenization should occur in short bursts to avoid breaking of mitochondria cells b. Short bursts include 3 reps x 5 seconds 4. Other options for homogenization, based on availability a. Homogenized in square-form beaker with polytron at 50% full speed for 2 x 10 seconds b. Pre-cooled mortar and pestle 5. Homogenate is filtered through two layers of cheesecloth into beakers cooled in ice. Cheesecloth is squeezed to ensure removal of most of the fluid 6. pH of filtrate is adjusted to 7.2 - 7.5 by drop-wise addition of 8 M KOH using one of the following a. narrow range pH paper b. pH meter 7. Filtrate divided equally into 50 ml conical bottom centrifuge tubes a. Amount of tubes dependent on starting tissue and grinding medium 8. Centrifuge bottles are balanced and centrifuged for 2 minutes at 5200 g in a centrifuge (CP&P Lab) at 2°C 9. Supernatant fraction decanted into clean, cold centrifuge bottles via pouring; do not transfer any of the pellet a. Pellet discarded in accordance with EH&S policies outlined in Waste Disposal section 10. Supernatant in clean tubes spun at 19,250 g for 5 minutes a. Resulting supernatant discarded via pouring into waste container i. Supernatant discarded in accordance with EH&S policies outlined in Waste Disposal Section 11. Crude mitochondria pellets washed with 5 ml of wash medium a. Eppendorf micropipette, 500-5000µl size, is used b. Pellet re-suspended via glass stirring rod i. Leave behind small white mass (this is starch) c. Pool the resulting crude mitochondrial solution into one tube per trial i. Resulting small white starch discarded in accordance with EH&S policies outlined in Waste Disposal section 12. Centrifuge at 5200 g for 2 minutes. a. Pipette supernatant into clean 50 ml, conical bottom centrifuge tubes while leaving behind slimy film i. Eppendorf micropipette used ii. Slimy film discarded in accordance with EH&S policies outlined in Waste Disposal section 13. Underlay each centrifuge tube with 8 ml of sucrose media a. Using an Eppendorf 5000 µl micropipette, draw 5 ml of sucrose solution b. Insert pipette into centrifuge tube, all the way to the bottom c. Dispense sucrose solution in an even motion, being careful to not press any air bubbles into the liquid. Once all sucrose is dispensed, keep button depressed (to not draw any liquid back up) while taking the pipette out of solution d. Change the setting on the pipette to 3 ml, and repeat a - c 14. Centrifuge resulting sucrose cushion and wash medium at 10,400 g for 20 minutes 15. Aspirate supernatant, leaving behind mitochondrial pellet 16. Re-suspend mitochondrial pellet with suspension medium and place on ice for future use Personal Protective Equipment / Engineering Controls: Nitrile gloves Safety glasses Face shield Dust mask Latex gloves Splash goggles Lab coat Fume hood Neoprene gloves Vented goggles Apron Biosafety cabinet Insulated gloves Eye wash station Safety shower Respirator Note: Open-toed and heeled shoes are NOT allowed. Skin - Wear protective, nitrile gloves and a laboratory coat. Avoid contact with skin and clothing. If exposure to skin occurs, wash immediately with non-abrasive soap and water. Eyes - Wear safety (or splash when using KOH) goggles to avoid exposure to eyes. If exposure to eyes occurs, remove any contact lenses and immediately flush with water for 15 minutes. If swallowed - do not induce vomiting. Give large quantities of water to dilute any/all chemicals. Seek medical attention. Handling & Storage Precautions: Keep all containers tightly closed and in a dry, cool, well-ventilated area. Avoid sources of ignition and flammable products. In addition, refer to MSDS(s) for each chemical’s specific conditions. Waste Disposal Procedures: Unless EH&S specifically instructs otherwise, all chemical/reagent waste (including excess solutions) must be placed in an appropriately labeled hazardous waste container for EH&S disposal. Compatible substances may be combined into one waste container. Spill/Release Containment and Clean Up/Decontamination Procedures: Accidental Release - KOH: Absorb neutralized caustic residue on clay, vermiculite or other inert substance and package in a suitable container for disposal. Try to avoid creating dust. Accidental Release - ALL: Ventilate area of leak/spill, avoid ignition, clean and expose in accordance with EH&S, and wash contaminated area with water. Health & Safety Summary for Required Reagents: C a r c i n o g e n T e r a t o g e n M u t a g e n R e p r o d u c t i v e E f f e c t s S e n s i t i z e r I r r i t a n t T o x i c Chemical name Mannitol X EGTA X X MOPS X X H i g h l y T o x i c C o r r o s i v e C o m b u s t i b l e Target Organ(s) May cause irritation to eyes and skin Toxic in large amounts by ingestion Very hazardous in case of eye contact; toxic to lungs BSA Cysteine X X KOH X X 2-mercapthoethanol X X Hazardous in the case of skin/eye contact, ingestion and inhalation Severe irritant of eyes and skin. Toxic Toxic; harmful if swallowed. X X C o m p r e s s e d G a s E x p l o s i v e F l a m m a b l e O r g a n i c P e r o x i d e s O x i d i z e r P y r o p h o r i c U n s t a b l e W a t e r R e a c t i v e H F e l a a l m t m h a b il it y Incompatibilities Strong Oxidizers; avoid heat, flames, and ignition sources 1 1 0 Keep away from heat and sources of ignition 2 1 0 Strong oxidizers and bases; Keep away from heat and sources of ignition 2 1 0 Strong acids and oxidizing agents 1 1 0 May be combustible at high temps. Avoid sources of ignition; evaporate residue under fume hood; avoid strong oxidizers 1 1 0 Corrosive; avoid contact with water, acids, flammable liquids. Avoid heat and moisture. Oxidizing agents and moisture. Avoid contact with metals, heat, and ignition sources. 3 0 2 The above summary consists of guidelines for proper handling & disposal of chemicals used in this procedure. You must read and understand the contents of the entire MSDS(s) before starting this procedure. References: Influence of Trinexapac-Ethyl on Respiration of Isolated Wheat Mitochondria. Heckman, Elthon, Horst and Gaussoin. Crop Science (2002) 42:2: 423-427 Acclimation, Hydrogen Peroxide, and Abscisic Acid Protect Mitochondria against Irreversible Chilling Injury in Maize Seedlings. Prasad TK, Anderson MD, Stewart CR. Plant Physiology (1994) 105: 619 - 627 MSDS sheets (on file) R e a c t i v i t y 3 2 1