Chem 2.3 WS
advertisement

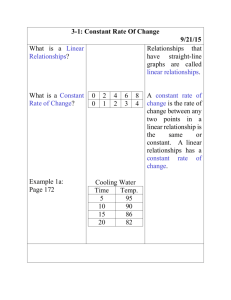
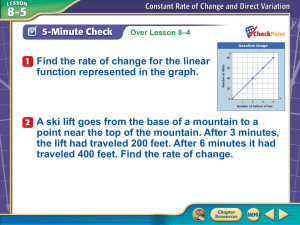
Name Date Class Gas Behavior You take a sealed, air-filled balloon, squeeze it and then release it. Next you take the balloon and place it in a refrigerator for half an hour, then remove it. In each case, explain the changes in volume, pressure, and temperature. 61D Name Date Class Gas Behavior Understanding Main Ideas If the statement is true, write true. If the statement is false, change the underlined word or words to make the statement true. 1. If the temperature of a gas is constant, when the pressure is increased, the volume decreases. 2. If the air pressure inside an inner tube is constant, when the temperature of the air is increased, the volume decreases. 3. The graph of the relationship between the volume of a gas at constant temperature and its pressure is a(n) line. 4. If the temperature of a gas inside a sealed, rigid container is decreased, its pressure decreases. 5. The graph for Charles’s law shows that the volume of a gas at constant pressure is inversely proportional to its temperature. 6. If a gas at constant pressure inside a cylinder topped by a movable piston is heated, the volume of the gas will increase and push the piston outward. Building Vocabulary Fill in the blank to complete each statement. 7. When the graph relating two variables is a straight line passing though the origin, the variables are proportional. 8. According to law, when the pressure of a gas at constant temperature is increased, the volume of the gas decreases. 9. According to law, when the temperature of a gas is increased at constant pressure, its volume increases. 10. When the product of two variables is constant, the variables are proportional to each other. 61E Name Date Class Gas Behavior Read the passage and study the graphs below it. Then use a separate sheet of paper to answer the questions that follow the graphs. Grasping Gas Graphs You have examined the relationship between the temperature and volume of a gas, and between the pressure and volume of a gas. Suppose a scientist wants to determine whether the number of particles of a gas is related to its pressure. Data for this experiment can be collected by increasing the number of gas particles in a container with a constant volume and temperature, and measuring the pressure of the gas. The number of gas particles can be increased by pumping more gas into the container. Sample data from such an experiment are shown by the solid line in Figure 1. The dotted line shows how the resulting graph can be extended. The relationship between the number of particles of a gas and its volume at a constant temperature can be determined in a similar way. Data for this experiment can be collected by increasing the number of gas particles in a cylinder that has a movable piston and measuring the effect on the volume. The graph in Figure 2 shows sample data from such an experiment. 1. What is the manipulated variable in each graph? What is the responding variable? 2. What is the relationship between the number of gas particles and pressure? Are these two variables directly proportional or do they vary inversely? 3. What is the relationship between the number of gas particles and volume? Are these two variables directly proportional or do they vary inversely? 4. When the number of gas particles in a container with constant volume decreases, how will the pressure of the gas change? 5. When the number of gas particles at constant pressure increases, how will the volume of the gas change? 61F Name Date Class Gas Behavior Write the letter of the correct answer on the line at the left. 1. At constant temperature, when the volume of a gas is decreased, what happens to its pressure? A It decreases. B It will vary. C It increases. D It remains constant. 2. At constant pressure, when the temperature of a gas is decreased, what happens to its volume? A It decreases. B It will vary. C It increases. D It remains constant. 3. At constant pressure, how are the temperature and volume of a gas related? A They are inversely proportional. B They are directly proportional. C They are constant. D They are indirectly proportional. 4. If the sun shining through windows heats the air in a sealed room, what happens to the air pressure in that room? A It decreases. B It will vary. C It increases. D It remains constant. Fill in the blank to complete each statement. 5. When Martin pushes down on the handle of his bicycle pump, the air pressure within the pump . 6. law describes the relationship between a gas’s volume and pressure when its temperature is constant. 7. law describes the relationship between a gas’s temperature and volume when its pressure is constant. 8. At constant temperature, the pressure and volume of a gas are proportional. 9. At constant volume, when the temperature of a gas decreases, the pressure . 10. When the graph relating two variables is a straight line passing through the origin, the variables are proportional to each other.