AP Organic FRQ
advertisement

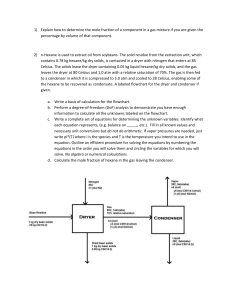
AP Organic FRQ: You have 20 minutes to do the following questions. You may use a calculator and the tables at the back of the book. The alkane hexane, C6H14, has a molecular mass of 86.17 g/mol. 1. Like all hydrocarbons, hexane will burn. Write a balanced chemical equation for the complete combustion of hexane. This reaction produces gaseous carbon dioxide and water. 2. The complete combustion of 10.0 g of hexane produces 487 kJ. What is the molar heat of combustion (ΔH) of hexane? 3. Determine the pressure exerted by the carbon dioxide formed when 5.00 g of hexane are combusted. Assume the carbon dioxide is dry and stored in a 20.0-L container at 27 °C. 4. Under identical conditions, hexane vapor diffuses at one-half the rate of the vapor of another compound. What is the molar mass of the other compound? 5. Hexane, like most alkanes, may exist in different isomeric forms. The structural formula of one of these isomers is pictured below. Draw the structural formula of any two other isomers of hexane. Make sure all carbon atoms and hydrogen atoms are shown. Answers and Explanations 1. 2C6H14 + 19O2→12CO2 + 14H2O Give yourself 2 points for the answer shown above, or for the coefficients: 1, 9/2, 6, and 7. Give yourself 1 point if you have one or more, not all, of the elements balanced. 2. (–487 kJ/10.0 g hexane) (86.17 g hexane/mol hexane) = –4.20 × 103 kJ/mol Give yourself 2 points for the above setup and correct answer (this requires a negative sign in the answer). If the setup is partially correct, give yourself 1 point. 3. The ideal gas equation should be rearranged to the form P–nRT/V. n – (5.00 g hexane) (1 mol hexane/86.17 g hexane) (12 mol CO2/2 mol hexane) = 0.3481 mol CO2 This answer has an extra significant figure. The mole ratio should match the one given in your balanced equation. You will not be penalized again for an incorrectly balanced equation. You will lose a point if you do not include a hexane-to-CO2 conversion. R = 0.0821 L atm/mol K This value is given in your test booklet. T = 27° + 273 = 300. K You will be penalized if your forget to use the Kelvin temperature. V = 20.0 L P = (0.3481 mol CO2) (0.0821 L atm/mol K) (300. K)/(20.0 L) = 0.429 atm. Give yourself 2 points for the correct setup and answer. Give yourself 1 point if you did everything correctly, except the mole ratio or the Kelvin conversion. 4. This part requires Graham's law: r1/r2 = (M2/M1)1/2 If we let "1" refer to hexane, and "2" refer to the unknown vapor, then: r1 = (1/2)r 2, and M1 = 86.17 g/mol. When these values are entered into the equation: Canceling and then squaring both sides gives: (1/2)2 = (1/4) = M2/86.17 g/mol M2 = (1/4)(86.17 g/mol) = 21.54 g/mol Give yourself 2 points for the correct setup and the correct answer. It is not necessary to show all the intermediate steps. Give yourself 1 point if you make a mistake. 5. You may need to redraw one or more of your answers to match the answers shown below. Give yourself 1 point for each correct answer, with a 2-point maximum. There are no bonus points for additional answers. These compounds are 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane and 2,3-dimethylbutane, respectively. These four, along with the original n-hexane, are the only isomers. If you think you have another isomer, you have simply redrawn one of these. Try naming your answer and see if it matches one of these names. Total your points. The maximum is 10 points. Subtract one point if all your answers do not have the correct number of significant figures.