Unit Concept Map
advertisement

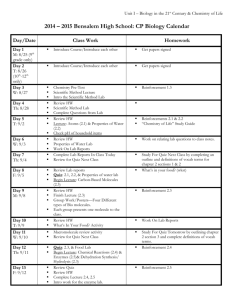
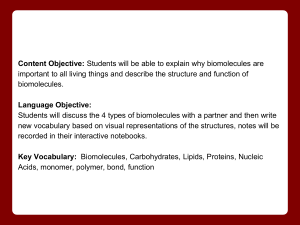
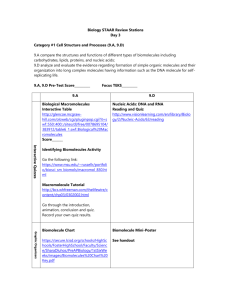
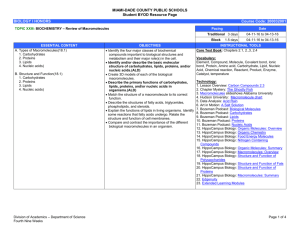
Unit 2 Concept Map Course Essential Question Unit 2 - The Chemistry of Biology: Life emerges due to the chemical organization of matter into cells. Grade Level: 10 Subject: Biology Unit Essential Question How does the structure of water and carbon-containing biomolecules enable organisms to carry out the chemical reactions that are needed to maintain homeostasis? PA Eligible Content BIO.A.2.1.1, BIO.A.2.2.1, BIO.A.2.2.2, BIO.A.2.2.3, BIO.A.2.3.1, BIO.A.2.3.2 Lesson Essential Questions How do the unique molecular and chemical properties of water enable the molecule to support life on earth? How does the tetrahedral atomic shape of carbon enable the element to be the backbone of biomolecules? How are carbohydrates, proteins, lipids and nucleic acids synthesized? How do environmental factors affect the synthesis of biomolecules? What are the classes of biomolecules and how do their structures relate to their functions within an organism? How do enzymes affect chemical reactions within all levels of organization and how do environmental factors affect their function? Concepts 1. 2. 3. 4. 5. 6. 7. Cells function as microscopic chemical factories synthesizing and degrading biological molecules necessary for life. Liquid water forms hydrogen bonds, is a solvent, and forms hydronium ions allowing a wide range of biochemical reactions to occur. Biological molecules produced by a cell can be used by the cell or transported outside for use by other cells. Cells are composed mostly of: C, H, N, O, P, and S. Carbon rings and chains form the backbone of all biological molecules. Many biological molecules are polymers made from monomers that contain carbon chemically bound with other elements. Carbohydrates, lipids, proteins, and nucleic acids are the chemical foundations for life. Molecular structure is related to function. 8. -----------------------------------------------------------------Skills 1. Analyze the unique properties of water and how these properties support life on earth (i.e. Vocabulary 1. Atom 2. Compound 3. Molecule 4. Van der Waals forces 5. Hydrogen bond 6. Cohesion 7. Adhesion 8. Suspension 9. pH scale 10. Monomer 11. Polymer 12. Carbohydrate 13. Monosaccharide 14. Lipid 15. Nucleic Acid 16. Nucleotide 17. Protein 18. Amino Acid 19. Organic molecule 20. Chemical reaction 21. Reactants 22. Biological macromolecules 23. Macromolecules 24. Catalyst 25. Isotope 26. Covalent 27. Ionic 28. Polar 29. Nonpolar 30. Solution 31. Enzymes 2. 3. 4. 5. 6. freezing point, high specific heat, cohesion) Explain how carbon is uniquely suited to form biological macromolecules Describe how biological macromolecules form from monomers Compare and contrast the structure and function of carbohydrates, lipids, proteins, and nucleic acids in an organism Describe the role on an enzyme as a catalyst in regulating a specific biochemical reaction Predict how changes in factors such as pH, temperature, and concentration levels affect enzyme function. Formative Assessments Summative Assessments 1. Ticket out the 1. Quizzes door 2. Unit Tests 2. Think-pair-share 3. Labs 3. Thumbs up – pH lab Thumbs down Temperature/enzyme 4. Concept Map - PH lab manual #2 5. Sentence starter Drops of water on a prompts Penny Lab 6. Collins writing 4. Projects 7. Venn diagram 5. CDT biology 8. Compare contrast 9. 3-2-1 10. Frayer diagrams 11. KWL 12. Whiteboard responses 13. Jigsaw 14. Chunking 15. Foldables 16. Text rendering 17. Guided reading 18. Whiparound 19. Pause-proceedlecture Resources Biology textbook Powerpoints (from textbook resources) Biology websites: -biologyjunction.com -biologycorner.com -biology.com