Topic IX – The Mole - Science - Miami
advertisement

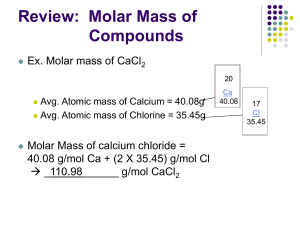
MIAMI-DADE COUNTY PUBLIC SCHOOLS Student BYOD Resource Page CHEMISTRY I Course Code: 200334001 TOPIC IX: The Mole Pacing Date Traditional 14 days Block 7 days Q2 Assessment ESSENTIAL CONTENT A. Develop Concept of the Mole 1. Atomic mass and formula mass 2. Definition of a mole 3. Avogadro’s Number 4. Molar mass B. Conversions with the Mole 1. Dimensional Analysis applied to the mole. a. Conceptual understanding of conversions b. SI Unit conversions i. Particles and moles ii. Mass and moles iii. Volume of a gas and moles. C. Empirical and Molecular Formulas Division of Academics – Department of Science Second Nine Weeks OBJECTIVES (Review) Interpret formula representations of molecules and compounds in terms of composition and structure. (Review) Predict the formulas of ionic compounds based on the number of valence electrons and the charges of the ions. Develop the concept of mole as a counting unit. Use formulas of compounds to find the formula mass and the molar mass. Use dimensional analysis to determine number of particles for elements and compounds using the mole concept, in terms of number of particles, mass, and the volume of an ideal gas at specified conditions of temperature and pressure. Use experimental data to determine, empirical formulas, molecular formulas 01-04-16 to 01-22-16 01-04-16 to 01-22-16 01-19-16 to 01-29-16 INSTRUCTIONAL TOOLS Core Text Book Chapter 3 pp60-99; Chapter 10 pp. 304 -343; Chap 15, Vocabulary: Atomic mass unit, Avogadro’.s number, ionic compound (formula unit), molecule, atom, gas, particle, element, volume, temperature, pressure, ideal gas, empirical formula, molecular formula, empirical formula, molar mass, mole, molecular formula Technology: 1. Khan Academy 2. http://www.pbslearningmedia.org/ (search your topic) 3. Chemistry Review Quizzes 4. QUIZ on Counting Particles and Avogadro’s Number 5. QUIZ Molar Mass 6. QUIZ Mole to Mass Conversions 7. QUIZ on Mass to Moles 8. Mole Notes 9. Progressive Science Initiative (PSI) a. http://www.njctl.org/courses/science/chemistry/molecalculations/ Page 1 of 3 MIAMI-DADE COUNTY PUBLIC SCHOOLS Student BYOD Resource Page CHEMISTRY I Standard: SC.912.N.3.5 Standard: SC.912.P.8.7 Course Code: 200334001 Video The Octet Rule and Atomic Bonding Chemical Bonding: Lewis and Resonance Structures Lewis Structures VSEPR's and Coulomb's Law Video Atomic Number, Atomic Mass, and Isotopes Empirical Formulas Atomic and Molecular Weights Formula Weight Mass Percent and Empirical Formulas What is Molecular Mass Math The Mass of Formula Units Explanation Video Standard: SC.912.P.8.9 Standard: MAFS.912.NQ.1.3 Molecular Mass and Molar Mass A Simplified Conversion Equation and Review Converting Grams to Moles What is Stoichiometery? Finding the Molar Mass of a Substance Avogadro's Number and the Mole Practicing with Avogadro's Number and the Mole: Example 3 Practicing with Avogadro's Number and Math the Mole: Example 1 Reaction Stoichiometry: Example 1 Explanation Step 1 Practicing with Avogadro's Number and the Mole: Example 2 Video Standard: MAFS.912.NQ.1.1 Calculating Molecular Mass and Formula Mass Division of Academics – Department of Science Second Nine Weeks Reaction Stoichiometry: Example 1 Step 2 Reaction Stoichiometry: Example 2 Reaction Stoichiometry: Example 3 Reaction Stoichiometry: Example 4 Formation Reactions Percent Composition Problems Based on Chemical Equations Combining Stoichiometry and Molarity Solutions in Chemical Reactions: Math Example One Explanation Solutions in Chemical Reactions: Example Two: Step One Video Molecular Geometry and Bonding Theories Polarity and Molecular Geometry Studying Molecular Structure Calculating Molecular Mass and Formula Mass Mole Fractions Concentration Solutions in Chemical Reactions: Example Two: Step Two Solutions in Chemical Reactions: Example Three: Step One Solutions in Chemical Reactions: Example Three: Step Two Solutions in Chemical Reactions: Example Three: Step Three Molarity Molality Page 2 of 3 MIAMI-DADE COUNTY PUBLIC SCHOOLS Student BYOD Resource Page CHEMISTRY I Course Code: 200334001 Experimenting with Molarity Math Finding Molarity: Example One Explanation Video Finding Molarity: Example Two Origami Chemistry: NYU Chemist Folds Molecules First-Ever Image of a Molecule (Million Times Smaller Than Grain of Sand) Quarks with Quirky Names Make Up Most Matter OK, Atoms, Say Cheese: Molecular Structures Photographed at Cornell Lab See Change: Physicists Have Color Films of Atoms for the First Time John Dalton: Atomic Symbols (1835) Image Division of Academics – Department of Science Second Nine Weeks Page 3 of 3