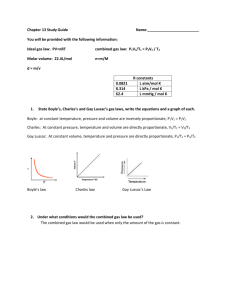
02.Gas Law CW 1 w ans
advertisement
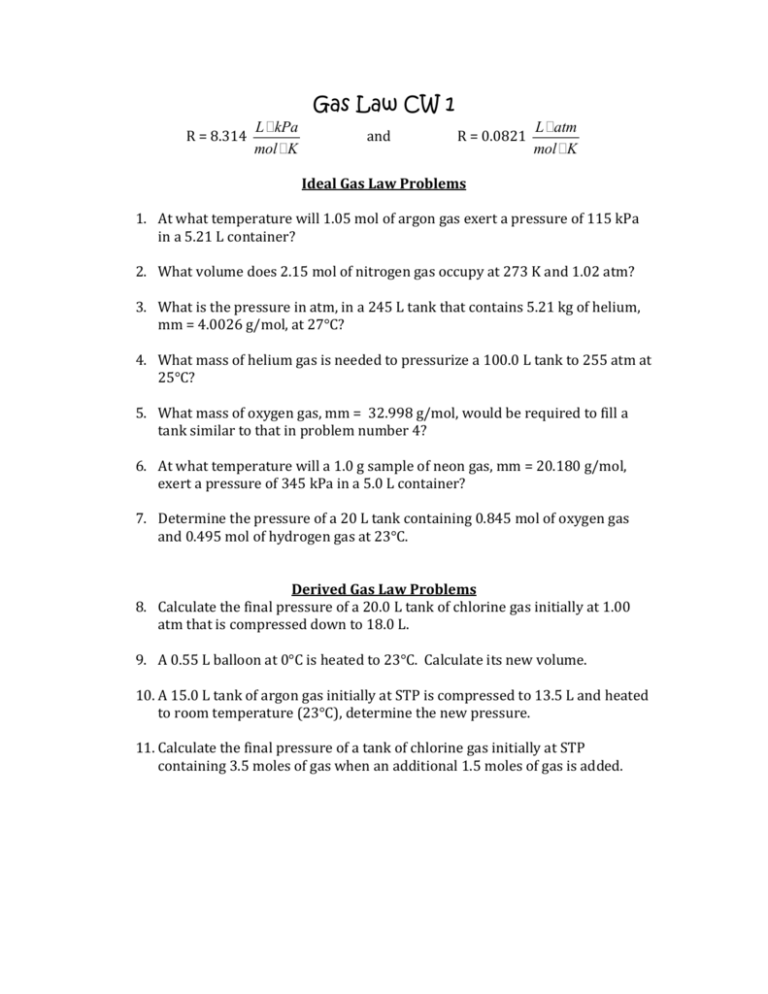
Gas Law CW 1 R = 8.314 L ikPa mol iK and R = 0.0821 L iatm mol iK Ideal Gas Law Problems 1. At what temperature will 1.05 mol of argon gas exert a pressure of 115 kPa in a 5.21 L container? 2. What volume does 2.15 mol of nitrogen gas occupy at 273 K and 1.02 atm? 3. What is the pressure in atm, in a 245 L tank that contains 5.21 kg of helium, mm = 4.0026 g/mol, at 27°C? 4. What mass of helium gas is needed to pressurize a 100.0 L tank to 255 atm at 25°C? 5. What mass of oxygen gas, mm = 32.998 g/mol, would be required to fill a tank similar to that in problem number 4? 6. At what temperature will a 1.0 g sample of neon gas, mm = 20.180 g/mol, exert a pressure of 345 kPa in a 5.0 L container? 7. Determine the pressure of a 20 L tank containing 0.845 mol of oxygen gas and 0.495 mol of hydrogen gas at 23°C. Derived Gas Law Problems 8. Calculate the final pressure of a 20.0 L tank of chlorine gas initially at 1.00 atm that is compressed down to 18.0 L. 9. A 0.55 L balloon at 0°C is heated to 23°C. Calculate its new volume. 10. A 15.0 L tank of argon gas initially at STP is compressed to 13.5 L and heated to room temperature (23°C), determine the new pressure. 11. Calculate the final pressure of a tank of chlorine gas initially at STP containing 3.5 moles of gas when an additional 1.5 moles of gas is added. Gas Law CW 1 Solutions 1. 2. 3. 4. 5. 6. 7. Ideal Gas Law Problems At what temperature will 1.05 mol of argon gas exert a pressure of 115 kPa in a 5.21 L container? (115) (5.21) = 1.05 (8.314) T T = 68.6K What volume does 2.15 mol of nitrogen gas occupy at 273 K and 1.02 atm? 1.02 V = 2.15 (0.0821) (273) V = 47.2 L What is the pressure in atm, in a 245 L tank that contains 5.21 kg of helium, mm = 4.0026 g/mol, at 27°C? 5210 / 4.0026 = 1302 mol He P (245) = (1302) (0.0821) (300) P = 131 atm What mass of helium gas is needed to pressurize a 100.0 L tank to 255 atm at 25°C? 255 (100.0) = n (0.0821) (298) n = 1042 moles (4.0026) = 4200 g or 4.20 kg What mass of oxygen gas, mm = 32.998 g/mol, would be required to fill a tank similar to that in problem number 4? 1042 (32.998) = 34.4 kg At what temperature will a 1.0 g sample of neon gas, mm = 20.180 g/mol, exert a pressure of 345 kPa in a 5.0 L container? 1 / 20.180 = 0.0496 mol 345 (5.0) = (0.0496) (8.314) T T = 4200 K Determine the pressure of a 20 L tank containing 0.845 mol of oxygen gas and 0.495 mol of hydrogen gas at 23°C. P (20) = 1.34 (0.0821) (296) P (20) = 1.34 (8.314) (296) P = 1.63 atm 165 kPa Derived Gas Law Problems 8. Calculate the final pressure of a 20.0 L tank of chlorine gas initially at 1.00 atm that is compressed down to 18.0 L. P1V1 = P2V2 (1.00) (20.0) = P2 (18.0) P2 = 1.11 atm 9. A 0.55 L balloon at 0°C is heated to 23°C. Calculate its new volume. V1 / T1 = V2 / T2 0.55 / 273 = V2 / 298 V2 = 0.60 L 10. A 15.0 L tank of argon gas initially at STP is compressed to 13.5 L and heated to room temperature (23°C), determine the new pressure. P1V1 / T1 = P2V2 / T2 (1.00)(15.0) / 273 = P2(13.5) / 298 P2 = 1.21 atm 11. Calculate the final pressure of a tank of chlorine gas initially at STP containing 3.5 moles of gas when an additional 1.5 moles of gas is added. P1 / n1 = P 2 / n2 1.00 / 3.5 = P2 / 5.0 P2 = 1.4 atm