Chapter 13 Study Guide Name: You will be provided with the
advertisement

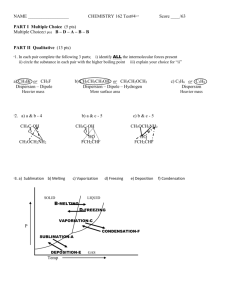
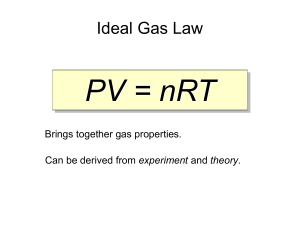
Chapter 13 Study Guide Name:_________________________ You will be provided with the following information: Ideal gas law: PV=nRT combined gas law: P1V1/T1 = P2V2 / T2 Molar volume: 22.4L/mol n=m/M d = m/v 0.0821 8.314 62.4 1. R constants L atm/mol K L kPa / mol K L mmHg / mol K State Boyle’s, Charles’s and Gay Lussac’s gas laws, write the equations and a graph of each. Boyle: at constant temperature, pressure and volume are inversely proportionate, P1V1 = P2V2 Charles: At constant pressure, temperature and volume are directly proportionate, V1/T1 = V2/T2 Gay Lussac: At constant volume, temperature and pressure are directly proportionate, P1/T1 = P2/T2 Boyle’s law Charles law Gay Lussac’s Law 2. Under what conditions would the combined gas law be used? The combined gas law would be used when only the amount of the gas is constant. 3. A weather balloon is filled with helium that occupies a volume of 5.00 X 104L at 0.995 atm and 32.0oC. After it is released, it rises to a location where the pressure is 0.720 atm and the temperature is -12.0oC. What is the volume of the balloon at the new location? V2 = P1V1T2 / T1P2 = (0.995 atm) (5.00 X 104L) (-12 + 273) / (32+273) ( 0.720) = 5.9 X 104L 4. State Avogadro’s principle. Equal volumes of gases at the same temperature and pressure contain equal number of particles. At STP, 1 mole of gas = 22.4L. 5. What volume of propane would 0.540 mol occupy at STP? 0.540 mol X 22.4 L / mol = 12.1 L 6. State the ideal gas law. The ideal gas law describes the physical behavior of an ideal gas in terms of pressure, volume, temperature and number of moles present. It assumes that the gas particles have no attraction for each other or another substance, that the gas particles have no volume of their own and that all collisions are elastic. 7. Calculate the number of moles of O2 gas held in a sealed, 2L tank at 3.5 atm and 25oC. PV = nRT n = PV / RT = (3.5 atm)(2L) / (0.0821)(25 + 273) = 0.29 mol 8. A 2 L flask is filled with ethane gas (C2H6) from a small cylinder at a pressure of 1.08 atm and a temperature of 15.0oC. What is the mass of ethane? PV = nRT, n = m/M , PV = mRT / M m = PVM / RT = (1.08 atm) (2L) (30g/mol) / (0.0821)(15 + 273) = 2.9g 9. What is the density of a sample of nitrogen gas (N2) that exerts a pressure of 5.30 atm in a 3.50 L container at 125oC? PV = nRT, n = m/M, d=m/v, m=PVM / RT = (5.30atm)(3.50L)(28g/mol) / (0.0821)(125+273) = 15.9 g d = 15.9g/3.5L = 4.5g/L 10. List the three conditions under which a gas is least likely to behave ideally. 1. extreme temperatures and pressures 2. when the molecular weight of the gas particles are large 3. polar gases 11. Using the following reaction, how much water vapor, in grams, would be produced from 6.5L of H2S at 2.0 atm and 290K? 2H2S + O2 2H2O + 2S 6.5L H2S X 2L H2O / 2L H2S = 6.5L H2O PV = nRT, n=m/M m=PVM / RT = (2.0 atm)(6.5L)(18g/mol) / (0.0821) (290K) = 9.8g H2O 12. Methane, CH4, undergoes complete combustion by reacting with oxygen gas to form carbon dioxide and water. a. Write a balanced, chemical equation for this reaction. CH4 + 2O2 CO2 + 2H2O b. How many liters of water vapor would be produced from 7.0L of CH4? 7.0L CH4 X 2L H2O / 1L CH4 = 14L H2O 13. Nitrogen and hydrogen react to form ammonia, NH3. How many liters of ammonia gas can be formed form 13.7L of hydrogen? N2 + 13.7L H2 X 3H2 2NH3 2L NH3 / 3L H2 = 9.1L NH3