The Chemistry of Natural Water
advertisement

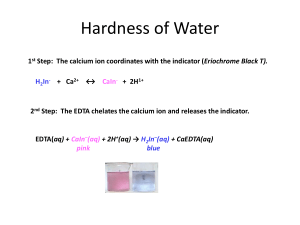
The Chemistry of Natural Water Lauren Kenney Chemistry 111 November 14, 2012 TA: Tammy Badger 1 Introduction Water hardness is important because it not only is a huge part of water quality, but it also has a big affect on both life and industry. Since all living creatures need water to survive, it is necessary to understand the chemistry behind natural water. The purpose of this experiment was to test several different water samples to determine the hardness, find out what makes the samples hard, and test a common water softening technique. Water hardness is defined as the sum of both the calcium and magnesium concentrations, expressed as calcium carbonate in milligrams per liter. Water is considered hard when there are high concentrations of the divalent cations Magnesium and Calcium and water is considered soft when there is a low combined concentration of Calcium and Magnesuim1. Hard water causes scale formation, large build-ups of calcium carbonate, that narrows pipes and is very costly to remove, which causes appliances like laundry machines and dishwashers to break easily, and when hard water is evaporated it leaves behind white residue. Hard water also effects how well soaps work. The soap reacts with the metal ions in hard water instead of the dirt on the surface being cleaned and produces a scum. Water softening agents such as washing soda and lime are used to slow down the scale build-up. They work by precipitating the Calcium and Magnesium ions as insoluble salts which are then removed by filtration or settling2. General guidelines for classification of waters are: 0 to 60 mg/L (milligrams per liter) as calcium carbonate is classified as soft; 61 to 120 mg/L as moderately hard; 121 to 180 mg/L as hard; and more than 180 mg/L as very hard3. Water hardness depends on what the water had contact with for example rainwater flows 2 over rocks dissolving some of the elements of the rocks. Areas rich in limestone and chalk tend to have hard water because the minerals are more soluble, while soft water usually comes from areas with granite and other impermeable rocks5. The four water samples for this experiment were obtained from various lakes and creeks around Pennsylvania and Ohio including Lake Moshannon, Loyalsock Creek, Spring Creek, and a test pit in Wilmington, Ohio. All of these samples come from lakes and creeks which get the main supply if water from rain or springs. While the majority of the samples will be classified as soft to moderately hard, they will be rank ordered on which is the hardest out of all four samples. Small streams and springs feed Lake Moshannon so this water sample should be the second hardest out the all the samples because the springs send water from under ground where there are many minerals and the streams flow into the lake picking up cations on the way, and the entire body of water sits over rock and sand6. Loyalsock Creek is mostly gets it’s water from rain, however the underlying geology is interbedded sediment and sandstone7. Since sandstone is soluble in water, it is likely that the flowing water of the creek will also be mildly softer than Lake Moshannon due to the ions that it picks up from the sandstone. The water sample from Spring Creek should be the hardest water out of the four samples because the underlying bedrock of Nittany Valley and Penn’s Valley is limestone8. Limestone is very soluble in water and the endless flowing of the creek dissolves the sediment picking up metal ions. The water from Wilmington Ohio test pit should be the third hardest sample in between Lake Moshannon and Loyalsock Creek. The geology of Ohio is very similar to the geology in Pennsylvania where the other three samples 3 are from and the sample sits in a pit for a period of time collecting dissolved cations. Atomic Absorption Spectrophotometry (AA) and ethylenediaminetetracetic acid (EDTA) titration were used to determine the hardness for the water samples in this experiment. AA works by using Beer’s Law that states that absorbance and concentration are directly related. EDTA is an inexpensive way of calculating water hardness and works by reacting with all divalent cations and not just calcium and magnesium. In a titration method, the EDTA complexes with Magnesium and Calcium and the indicator eriochrome black T (EBT). The water will turn blue if it is soft1. In AA only the specific concentration of calcium and magnesium divalent ions are tested for. The AA method is indeed more expensive, however it is extremely accurate. The water sample is aspirated into a flame in the device and a light beam is directed at the flame onto a detector, which measures the amount of light absorbed by the aspirated water. Since each element has a unique wavelength, the source lamp is made of the element (calcium or magnesium). The amount of energy that the wavelength absorbed by the flame is proportional to the concentration of the element in the sample over a limited concentration range4. It is expected that the hardness of each sample rank in this order (from softest to hardest): Loyalsock Creek, Wilmington Ohio, Lake Moshannon, and Spring Creek. Procedure A water sample was taken from each of the following locations: Spring Creek, Lake Moshannon, Loyalsock Creek, and Wilmington, Ohio. Each group member took 4 a sample and performed a series of experiments including AA and EDTA titration to determine the hardness of the sample. Atomic Absorption Spectroscopy was performed by shinning a monochromatic light though the sample to measure the amount of light absorbed. According to Beer’s Law, the amount of absorbance is directly related to the concentration. Two calibration graphs and equations were created from data points obtained by testing known concentrations of calcium and magnesium, recording their absorbance, and creating a trend line for the data. Each water sample was then put into each of the machines and the absorbance was read off the machines. The data was applied to the specific calibration line equation and the hardness of each sample was calculated. EDTA titration was performed on each water sample. One drop of NH3/NH4CL/MgEDTA, EBT indicator, and the water being tested were added to each well of a 1x12 strip. Then a serial titration was performed and the EDTA solution was titrated across the well strips so that the first well had 1 drop and the last well had 12 drops. The first well that turned blue after the titration was recorded. The number of EDTA drops in that well were used to determine the hardness of that water sample by equations seen in the results section. A more detailed procedure can be found in PSU Chemtrek: Small-Scale Chemistry for General Chemistry. Written by Stephen Thompson, edited by Joseph T. Keiser 2012-2013, published by Hayden McNeil, and located on pages 10-1 to 10-221. 5 Results Figure 1: AA calibration graph for Ca2 Absorbance (Ca2+) vs Concentration (ppm) an 427.7nm 0.5 y = 0.0081x + 0.0089 R² = 0.9989 Absorbance 0.4 0.3 0.2 0.1 0 0 5 10 15 20 25 30 Concentration 35 40 45 50 Figure 2: AA calibration graph for Mg 2+ Absorbance (Mg2+) vs Concentration (ppm) at 202.5nm 0.5 y = 0.0142x + 0.0227 R² = 0.9961 Absorbance 0.4 0.3 0.2 0.1 0 0 5 10 15 20 Concentration 25 30 35 6 Table 1: AA hardness results for Lake Moshannon water sample Absorbance ppm hardness Calcium 0.0508 12.9 Magnesium 0.0329 7.16 Total Hardness 20.06 The calibration line equation in Figure 1 (y =0.0081x + 0.0089) was used to calculate the concentration in ppm. The Ca2+ absorbance was substituted for the y variable to solve for x. 0.0508=0.0081x+0.0089 0.0419=0.0081x x = 5.17 ppm Ca2+ To get ppm CaCO3, it is necessary to multiply the concentration by the molar mass of CaCO3 divided by the molar mass of Ca2+. CaCO3 5.17 ppm Ca2+ * ((100g CaCO3/ 1mole)/(40.0g Ca2+/ 1mole)) = 12.9 ppm The same process was used to calculate concentration in ppm for Mg2+. The calibration line equation in Figure 2 (y=0.0142x + 0.0227) was used to calculate the concentration in ppm of Mg2+. The absorbance was substituted for the y variable to solve for x. 0.0329 = 0.0142x + 0.0227 x = 0.718 ppm Mg2+ To convert that concentration to ppm of CaCO3, multiply the concentration by the molar mass of CaCO3 divided by the molar mass of Mg2+. CaCO3 0.718n ppm Mg2+ * ((100g CaCO3/ 1 mol)/(24.3g Mg2+/ 1mol)) = 2.96 ppm Table 2: AA hardness results for all water samples Absorbance units ppm hardness .0508 .0329 12.9 2.96 15.86 .0417 .0139 10.1 0 10.1 Lake Moshannon Ca2+ Mg2+ Total Hardness: Loyalsock Creek10 Ca2+ Mg2+ Total Hardness: Spring Creek11 7 Ca2+ Mg2+ .3263 .2957 195.9* 158.2* 354.1 .0435 .0181 10.7 0 10.7 Total Hardness: Wilmington Ohio12 Ca2+ Mg2+ Total Hardness: * Concentration multiplied by 2 due to dilution Table 3: EDTA Titration results for Lake Moshannon water sample Drops of EDTA ppm hardness Water sample 2 40 Softened sample 1 20 To convert the drops of EDTA to ppm hardness we first must figure out the concentration of the divalent cations using the equation (MEDTA VEDTA = MCations VCations)1 2*10-4 * 2 drops = MCations * 1 drop 4*10-4 = MCations To covert the concentration of CaCO3 to hardness we must multiply by CaCO3 molar mass and then convert it into mg CaCO3: (4*10-4 mol CaCO3/ liter) * (100 g CaCO3/ mol CaCO3) * (1000mg CaCO3/ 1g CaCO3) = 40 mg CaCO3/ liter 40 mg CaCO3/L =40 ppm hardness because the density of water is 1000g/L and 1ppm = 1 mg CaCO3/1000g water. To convert the ppm into grains/gallon, multiply the hardness by the conversion factor: (1 grain CaCO3 / gallon) is equivalent to 17.1 ppm 40ppm *(1 grain per gallon /17.1ppm) = 12 grains per gallon Table 4: EDTA hardness for all water samples Drops EDTA Lake Moshannon: Water Sample 2 Loyalsock Creek10: Water Sample 6 11 Spring Creek : Water Sample 2 12 Wilmington Ohio : Water Sample 11 * Concentration multiplied by 2 due to dilution ppm hardness/grains per gallon 40/2.34 120/7.02 80*/4.68 220/12.9 8 Discussion The results from the AA testing do not reject the hypothesis, however the results from the EDTA titration do indeed reject the hypothesis. According to the AA results (Table 2), Spring Creek has the hardest water sample of 354.1 ppm because the creek flows over very permeable and water-soluble rocks like limestone and dolomite and most of the water comes from underground springs8. Lake Moshannon has the second hardest sample because it also gets water from underground springs and streams but the water does not flow over soluble rocks, it just sits for a long period of time6. The next hardest sample came from Wilmington Ohio in a test pit. The area mainly gets water from underground wells and springs so there still is a presence of dissolved cations since the geology of Ohio is similar to the geology of Pennsylvania with an abundance of soluble bedrock like limestone and dolomite9. The softest sample was from Loyalsock Creek because the creek gets most of its water from rain but it still flows over water-soluble rock sediment7. Since the AA is much more accurate, there are many different errors that could have happened during the EDTA titration and calculation process. Perhaps the number of drops of EDTA was miscounted causing the solution to change color earlier or later than expected. This is important because for each well is an additional 20ppm so that could be significant if the drops were more or less than what they were supposed to be. Another reason is that four different people tested one water sample and one group member’s perception of when the well turned blue might be different from when another group member thought the well was 9 completely blue, which affected the accuracy. The main reason why the EDTA results are significantly higher than the AA results is because EDTA reacts with all of the cations instead of just Ca2+ and Mg2+. AA is done by a machine and has a high accuracy and precision, while the precision for EDTA titration was high due to the fact that the same well turned blue during both trials. For some group members, the sample did not turn blue in the same well both times which negatively impacted the precision and could have caused flawed data. One issue with AA was that equation for the line of the Mg2+ Concentration vs. Absorbance graph (Figure 2) gave negative numbers when solving for the concentration two of the samples. This means that the concentration of magnesium is so little that it is not even relevant to the total hardness so it ends up to be zero. The AA works the best because it only tests for the calcium and magnesium concentration, which is important because calcium and magnesium contribute the most to hardness and scale formation in industrial use of water, water softening only affects those two cations, and it is a much quicker and easier process (but much more expensive). Although only the results of one sample treated with a softening agent are shown (Table 1), all of the samples were significantly softer after being treated with the softening agent. Conclusion Water hardness is not only important for personal use, but it also has a big impact on industrial water use as well. Water that comes from underground springs and flows over highly soluble rocks is much harder than typical stream water due to the fact that it is in contact with the soluble sediment longer and has more time to 10 pick up the dissolved cations. While stream water does still flow over water-soluble rock, it still mostly comes from rain and is therefore softer. Water softening agents are one common way to treat water to reduce hardness and get better personal and industrial use. The type of rock that the water comes in contact with and the source of water are the biggest factors when considering water hardness. Reference 1PSU Chemtrek: Small-Scale Experiments for General Chemistry. 2012-2013. Stephen Thompson. Published by Hayden McNeal. Edited by Joseph T. Keiser. Experiment 10: The Chemistry of Natural Water. Pg. 10.1-10.22. 2Spellman, Frank R: The Science of Water Concepts and Applications, Second Edition; CRC Press, 2007, 289-304. 3"Water Hardness and Alkalinity." USGS Water-Quality Information:. U.S Geological Survey, 13 Apr. 2012. Web. <http://water.usgs.gov/owq/hardness-alkalinity.html>. 4Trimble, Stanley W: Encyclopedia of Water Science, Second Edition; CRC Press, 2007, 1327-1336. 5Thalmanand, Katherine L, Bedessem, James M..Fierro, Pedro Ed: The Water Encyclopedia, Third Edition Hydrologic Data and Internet Resources; CRC Press,2007, 8.1-8.218. 6"Black Moshannon State Park." PA DCNR . Pennsylvania Department of Conservation and Natural Resources, 2012. Web. <http://www.dcnr.state.pa.us/stateparks/findapark/blackmoshannon/index.htm>. 7"Loyalsock Creek Watershed Profile." Loyalsock Creek Watershed Profile. Susquehanna River Basin Commission, 2011. Web. <http://mdw.srbc.net/remotewaterquality/watershed_profiles/loyalsock.htm>. 8"The Spring Creek Watershed." The Susquehanna River Basin Hydrologic Observing System:(SRBHOS). Penn State, 2011. Web. <http://www.srbhos.psu.edu/srb_testbeds/springcreek.asp>. 9"Wilmington, Ohio Water Department." Wilmington, OH. City of Wilmington, 2009. Web. <http://www.ci.wilmington.oh.us/water.cfm>. 10Loyalsock Creek Water Sample: Kaylene Killeen Lab Notebook. 11 11Spring Creek Water Sample: Cooper Lacasse Lab Notebook. 12Wilmington, Ohio Water Sample: Scott King Lab Notebook. 12