Periodic Trends Report
advertisement

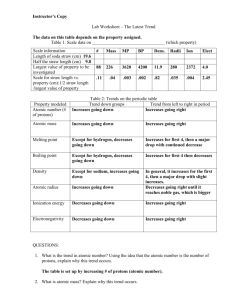
Brook H. Rollins V, Jordan Davis, Evan Sova 8-16-12 Dr. Metzker Chem 3010 Trends and Characteristics of the Periodic Table The ionization energy of an atom is the energy required to remove electrons to form cations. Which is in direct relation to the atomic radii and orbital configuration. As the atomic radii decreases across a period, the ionization energy increases. This is due to the atom’s smaller size, which pulls electrons closer to the nucleus, making it harder to remove the electrons. But when electrons are added (such as moving down a group or to an additional sub shell) the atomic radii increases, making outer electrons easier to be removed. Exceptions are boron and mercury, because removal of one of these outer electrons actually results in making the atoms unstable and higher ionization energy. Beryllium and boron are some exceptions to the general ionization trend. According to the trend boron should technically have a higher ionization than that of beryllium. This case is caused by beryllium having a full 2s subshell causing it to be stable. In boron the 2p electron is easier to remove than the stable 2s in beryllium. Figure 1 shows the general trend for ionization trend. Figure 1-Periodic trend for Ionization Energy Electron affinity is an amount of energy released by an atom when it becomes ionized; it increases across a given period due to an increase in the valence electrons. The trend of electron affinity is increasing from left to right across the period and increasing from top to bottom down a group. Exceptions to these trends generally are found in group 1A to group 2A and group 5A to group 6A. Both 1A and 5A have a higher electron affinity than their following group. This is caused by the stability created by having subshells (s and p) that are half full. This causes the energy of these groups to be higher than expected. The general trend is shown in Figure 2. Figure 2- Electron Affinity General Trend Electronegativity is a measure of the ability of an atom to draw an electron to itself. Electronegativity increases across a given period due to the increasing effective nuclear charge. The effective nuclear charge is an attractive force between the electrons in the electron cloud and its positively charged nucleus. While this value increases, attracting electrons becomes more favorable for attachment of the atom. The electronegativity trend also decreases down the periodic table due to increasing atomic radii; therefore decreasing the effective nuclear charge and making it less favorable for an atom to attract an electron. The exception to the trend is Argon, because Argon is not believed to form bonds. The periodic trend is shown in Figure 3. Figure 3- Electronegativity Periodic Trend The atomic radii is very easy to see and spot out on the periodic table, but sometimes the empirical value is somewhat skewed. By definition of atomic radii, it is the size of the isolated neutral atom and the length is measured in angstroms (Å). The general trend for atomic radii found to be decreasing along the period and increasing down each group. Exceptions to the trend are the noble gases. The noble gases cannot be measured due to their extreme stability due to the octet being full. Another exception is the transitional metals in groups 12-15. This is caused by the electrons being added to the inner shell before the outer shell to increase shielding of the outer shell and thus increasing stability. The general trend is show in Figure 4. A graphic diagram is shown in Figure 5. Figure 4- Periodic Trend for Atomic Radii Figure 5- Graphic Trend for Atomic Radii The melting point versus the atomic number trend depicts that with increasing atomic number the melting points tend to increase. The reasoning for why the melting point fluctuates by increasing and decreasing with the increasing atomic numbers is due to the varying states of matter for each of the elements. For example, the first two elements on the periodic table, hydrogen and helium are both naturally occurring gases and the next few elements are metals, which significantly increase in melting point values. The reason for why metals have higher melting points are due to the high amount of energy needed to break bonds to change the solid phase of a substance to a liquid phase. The stronger the bond between the atoms of an element, the higher the energy required in breaking that particular bond. Temperature in this case is the energy being used to break the bond to change the solid to a liquid. Figure 6- Periodic Trend for Melting Point