Nernst Presentation Notes
advertisement

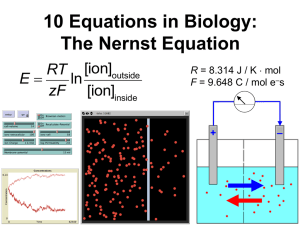
Seminar 3. Nernst Equation, part 1 Goals a. Informally derive an equation from prior scientific knowledge. (Faster than full derivation using word & formal equations, and more applicable to complex/unfamiliar systems, but less thorough.) b. Verbally analyze an equation to understand its organization and its inclusion of specific terms. (Identify and address gaps in students’ prior knowledge, and relate abstract concepts to physical interpretations.) c. Mathematically analyze an equation, and interpret the outcomes in terms of specific physical processes. (Shift focus from mechanical manipulation toward biological understanding, and emphasize that numeric outcomes must make biological sense.) Lesson Components 1. Brief mini-lecture on equilibrium potential. • concentration and electrical gradients • simple definition of eq. potential • review list of key concepts from brainstorming session 2. Small-group exercise: Identify specific parameters that should be part of the model, and whether each parameter has a positive or negative effect on equilibrium potential. Share with entire group. • Note: for now, focus on system that has just ONE ionic species. • Parameters: ion concentration on each side of membrane (+/- effect), ion charge (-), membrane permeability (0: affects kinetics but not equilibrium), system temperature (+) 3. Small-group exercise: Now write an equation that has the desired features. Demonstrate dimensional analysis as a way to assess an equation’s validity. • Higher temperatures (T) increase equilibrium potential E; greater positive charge of ion (z) decreases it. So E µT - z ? What units would this have? [Clearly, it doesn’t work!] How could we combine these parameters in a way that handles units more sensibly? [ E µT / z ] • Work time: at least 3–5 minutes. 4. Present the Nernst equation: E = RT ln [ion]outside , and analyze its qualitative interpretation. zF [ion]cell • Dimensional analysis. Give units for R and F. Review that 1 C = 1 As 6.241018 elementary charges such as electrons. On board: Plug in other units, then simplify. [ (J / K × mol ion) (K) (mol ion / L solute) : reduces to J/C.] Express this E= ln – – (mol e s / mol ion) (C / mol e s) (mol ion / L solute) verbally. [Amount of energy released by one coulomb of charge flowing between two points with a specific difference in electrical potential.] That diff. in potential is defined as 1 V. • Interpret R as amount of energy needed to heat 1 mol of an ideal gas by 1 degree Kelvin. Thus, R relates energy to temperature. • Why is this factor present in the ideal gas law PV = nRT ? [The IGL relates an ideal gas’s temperature T to the kinetic energy of its molecules: PV has units of energy.] Why is it present in the Nernst equation? [Nernst also relates T to the ions’ kinetic energy, which influences their diffusion rate.] • Interpret F as amount of electrical charge carried by 1 mol of electrons. Thus, F relates what two physical quantities? [Concentration to electrical charge.] 5. Analyze the Nernst equation’s quantitative behavior. • In step (4), you might have written [ion]outside – [ion]cell . How is the ln expression similar? [Both =0 when concentrations are equal.] How is the ln expression different? [Mathematically: units cancel. Physically: states that behavior is determined by the relative concentration difference, not the absolute difference. So 10 vs. 1 mmol is treated the same as 100 vs. 10.] • Under what conditions is E = 0? Why? [(i) T = 0: if there is no molecular motion, then no electrical field is needed to prevent ions from diffusing down their concentration gradient. (ii) [ion]outside = [ion]cell: if concentrations are equal, there is no net diffusion, so again no electrical field is needed to prevent it.] • Apply the Nernst equation to an uncharged solute such as O2. [z = 0, so E . According to the Nernst equation, electrical fields do not interact with uncharged solutes, and therefore cannot prevent their diffusion.] Is this interpretation realistic? [Probably not: Even solutes with no net charge may have localized charges that interact with the electrical field. Tradeoff within the model: simplicity vs. realism.] 6. Solve and interpret an example. • Human skeletal muscle: [Na+]cell 15 mM, [Na+]outside 145 mM. Calculate E[Na+]. [Participants must ask for values of R = 8.314 J/Kmol and F = 96,485 C/mol e–. This yields E[Na+] = 0.064 V = 64 mV.] • Interpret this result. [The sodium ion concentration is higher outside the cell than inside, so net diffusion rate would normally be inward. To prevent this, the cytoplasm must have a net positive charge to repel the positively charged sodium ions.] • How do cells create and maintain their membrane potential? [Ion pumps; e.g., Na+/K+ pump in animal cells, proton pump in many prok & euk cells. Relate also to proton pump in inner mitochondrial membrane of euk cells.]