09.Review to date
advertisement

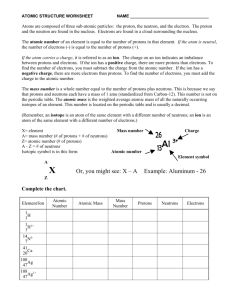
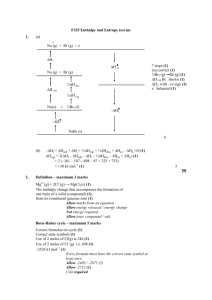
Review to date 1. How many protons and neutrons are in potassium-40? 2. How do cations form? 3. Identify the isotope containing 18 p+ and 20 n0 4. Calculate the atomic mass of V 50 V 49.947163 g/mol 0.250 % 51 V 50.943964 g/mol 99.750 % 5. Vocabulary to know: a. Isotope b. Atomic number c. Atomic mass d. Mass number e. Ion f. Cation / Anion g. Polyatomic ion 6. How many protons and electrons are in Y+3 7. What occurs within a neutral sulfur atom to form its common ion? 8. Calculate the atomic mass of Cl 35 34.968853 g/mol 75.78% 37 36.965903 g/mol 24.22% Cl Cl 9. Write the symbol for the isotope of Iron with 30 neutrons 10. Form compounds from the following cation / anion pairs a. Ca+2 and PO4-3 b. Al+3 and OHc. P-3 and NH 4+ Review to date solutions 1. How many protons and neutrons are in potassium-40? 19 p+ and 21 n0 2. How do cations form? When elements lose electrons Ar 3. Identify the isotope containing 18 p+ and 20 n0 38 18 4. Calculate the atomic mass of V 50 V 49.947163 g/mol 0.250 % 0.00250 0.1248679075 0.99750 50.81660409 51 V 50.943964 g/mol 99.750 % 50.94147 50.941 g/mol 5. Vocabulary to know: a. Isotope – elements with same # of p+ but diff # of n0 b. Atomic number – number of protons c. Atomic mass – weighted average of the isotopes of an element d. Mass number – sum of protons and neutrons e. Ion – element with a charge f. Cation / Anion – positive / negative ion g. Polyatomic ion – ion made of multiple elements 6. How many protons and electrons are in Y+3 39 p+ and 36 e7. What occurs within a neutral sulfur atom to form its common ion? It will gain two electrons 8. Calculate the atomic mass of Cl 35 Cl 34.968853 g/mol 75.78% 0.7578 26.4993968 37 Cl 36.965903 g/mol 24.22% 0.2422 8.9531417066 35.452538 35.45 g/mol 9. Write the symbol for the isotope of Iron with 30 neutrons 10. Form compounds from the following cation / anion pairs a. Ca+2 and PO4-3 Ca3(PO4)2 b. Al+3 and OH- Al(OH)3 c. P-3 and NH 4+ (NH4)3P 56 26 Fe