Periodic Table Trends Activity
advertisement

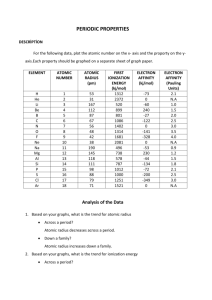
Name: _________________________________ Date: _______ Period: _____ Periodic Trends Description In this activity you are going to create graphs of illustrating the major periodic trends found in the modern periodic table. Below is a chart that contains information for the first 20 elements of the periodic table. You need to create a chart comparing the atomic number with one of the sets of data. You will plot the atomic number on the x-axis and the property, ex: atomic radius, on the y-axis. You will create 3 graphs each on a separate sheet of paper. Each graph must include: A proper title, labeled axes with consistent spacing and units for both axes Each data point labeled with the element symbol. (ex. Li) Data points should be connected by lines. Colored lines representing each period (ex: period 1 H, He would be yellow, period 2 Li, Be, B, C, N, O, F, Ne would be green, etc.). Make a key matching your chosen colors with the period. Graph 1: Atomic # (x-axis) vs. Atomic Radius (y-axis) Graph 2: Atomic # (x-axis) vs. 1st Ionization Energy (y-axis) Graph 3: Atomic # (x-axis) vs. Electron Affinity (y-axis) Element H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Atomic Number 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Atomic Radius (pm) 53 31 167 112 87 67 56 48 42 38 190 145 118 111 98 88 79 71 220 180 1st Ionization Energy (kJ/mol) 1312 2372 520 899 801 1086 1402 1314 1681 2081 496 738 578 787 1012 1000 1251 1521 419 590 Electron Affinity (kJ/mol) -73 0 -60 240 -27 -122 0 -141 -328 0 -53 230 -44 -134 -72 -200 -349 0 48 2 Glossary 1. Ionization Energy - this is defined as the amount of energy needed to remove an electron from an atom. The force attracting the electron increases as size decreases and as the positive charge increases. 𝑴 → 𝑴+ + 𝒆 − 2. Electron Affinity - this is defined as the amount of energy required to add an electron. 𝑿 + 𝒆 − → 𝑿− 3. Reactivity - this is defined as how readily an atom reacts. For metals this is based on ionization energy where the most reactive metals are those with the lowest ionization energies. For nonmetals this is based on electron affinity where the most negative electron affinity is the most reactive. This has to do with the fact that metals form positive ions and nonmetals for negative ions. Name: _________________________________ Date: _______ Period: _____ Analysis 1. Are there any elements that do not fit the periodic trends? Where are they in the periodic table? What properties do not fit the periodic trends? Why are they different? Atomic Radius 2. Define atomic radius in your own words. 3. What is the trend as you move down a group (column)? (think about the number of shells) Why? What is the trend as you move across a period (row)? (think about the number of protons and number of electrons in that shell) Why? 4. Arrange the following atoms in order of increasing atomic radius: potassium, carbon, rubidium, iodine, fluorine, and lithium. Explain your order. 5. When an atom loses an electron, what is its charge? What do you think happens to the size of the atom? Why? 6. When an atom gains an electron, what is its charge? What do you think happens to the size of the atom? Why? 7. Arrange the following ions in order of increasing atomic radius: S2-, Cl-, Ar, K+, Ca2+, and Br -. Explain your order. Ionization Energy (potential) 8. Define ionization energy in your own words. 9. What is the trend as you move down a group (column)? Why? What is the trend as you move across a period (row)? Why? Name: _________________________________ Date: _______ Period: _____ 10. Arrange the following atoms in order of increasing ionization energy: lithium, oxygen, magnesium, strontium, chlorine, and tellurium. Explain your order. 11. Using an activity series, what can you deduce about the relationship between ionization energy and reactivity of metals? Denisty 12. Define density in your own words. 13. How does density change as you move down a group (column)? Why? How does density change as you move across a period (row)? Why? 14. Some elements are found only in the gas state, for example Hydrogen is always found as, H2. How does the density of a solid compare with the density of a gas? Where is this seen in the periodic table aside from Hydrogen? How are the trends affected? Melting and Boiling Point 15. Define melting and boiling point in your own words. 16. How do the boiling points and melting points change as you move down a group (column)? Why? How do the boiling points and melting points change as you move across a period (row)? Why?