Lesson Plan 11-9-15 - Trousdale County Schools
advertisement

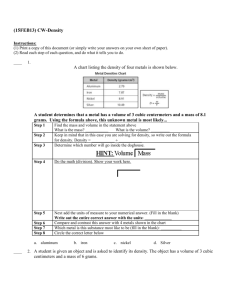
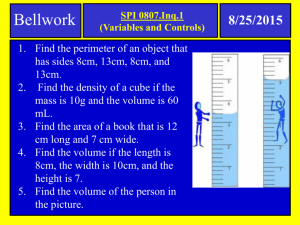
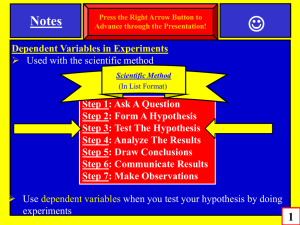
Trousdale County Schools Focused Lesson Plan 2015-16 Teacher: Joseph Randall White Unit Name: Chemical and Physical Reactions / Mixtures Unit #: 5 –Physical and Chemical Reactions Unit Length: 5 days Week: 11-9-2015 Week ___15__ of __36____ Subject: 8th Grade Science Tennessee State Standard(s) to be taught: (Write the entire standard) SPI 0807.9.1 Recognize that all matter consists of atoms. SPI 0807.9.3 Classify common substances as elements or compounds based on their symbols or formulas. SPI 0807.9.9 Use the periodic table to determine the properties of an element. SPI 0807.9.2 Identify the common outcome of all chemical changes. SPI 0807.9.10 Identify the reactants and products of a chemical reaction. SPI 0807.9.8 Interpret the results of an investigation to determine whether a physical or chemical change has occurred. SPI 0807.9.11 Recognize that in a chemical reaction the mass of the reactants is equal to the mass of the products (Law of Conservation of Mass). SPI 0807.9.4 Differentiate between a mixture and a compound. I Can Statements : I realize atoms make up all matter. I can tell the difference between an element and a compound by symbols and formula. I can determine the properties of an element using the periodic table. I can tell the difference between a chemical and physical reaction. I can explain the one common outcome of all chemical reactions. I can explain the difference between reactants and products. I can tell that the mass of the reactants should equal the mass of the products in any reaction. I can tell the difference between a mixture and a compound. Accommodations for students, both regular and special populations : Note for Students, peer-assistance, Unit Vocabulary: Volume, Mass, Density, Matter, Energy, Balance Scale, Immersion, Grams. Kinetic Theory, Thermal Energy, atom, proton, neutron, electron, Element, Atomic Number, Atomic Mass, Atomic Symbol, Isotope, Ion, Valance, Shell, Period, Group, Family, Reactivity, Malleable, Ductile, Conductive, Halogen, Alkali, Noble Gas, Metalloid, Transitional Metal, reactant, product, LOCOM, Mixture, Homogeneous, Heterogeneous, Pure Substance Resources, Technology, Formative and/or Summative Daily Agenda Assessments, Assignments, and a Daily Activity for citing text based evidence in conversations and/or writing Monday Bell ringer Test Review Textbook Reading Test over “Chemical Reactions” – Part #1 – 50 Interactive Power point projection questions Scaffolding for Students Tuesday Test over “ Chemical Reactions” Part #2 – 50 questions Grading Tests in class. Bell ringer Textbook Reading Interactive Power point projection Video Supplement Scaffolding for Students 1 Wednesday Warm- Up – Element Info Sheet Finish Grading Tests Most missed Questions on Test. Charting our progress through Chemistry Diagram Begin Mixtures ppt Bell ringer over Benchmark Assessment’s most missed questions. Interactive Power point Exit Ticket Textbook Reading Interactive Power point projection Video Supplement Scaffolding for Students Thursday Warm- Up – Name That Element Review Everything From Properties of Matter to LOCOM Mixtures ppt Mixtures Lab Friday Admin Day Bell ringer Exit Ticket Textbook Reading Interactive Power point projection Video Supplement Scaffolding for Students Bell ringer Exit Ticket Textbook Reading Interactive Power point projection Video Supplement Scaffolding for Students 2