CHEM 442 Lecture 20 Problems 20-1. Write down the electronic
advertisement

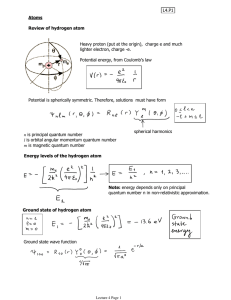
CHEM 442 Lecture 20 Problems 20-1. Write down the electronic Schrödinger equation of the helium atom. 20-2. Show that, with the neglect of electron-electron repulsion term in the Hamiltonian in 20-1, the separation of variables occurs. Find the exact energy and wave function of the Schrödinger equation of the helium atom with this approximate Hamiltonian. (In this problem only, the wave function does not have to satisfy the antisymmetry condition.) 20-3. Suggest the singlet wave function of the helium atom with both electrons in the 1s orbital within the orbital approximation. The wave function needs not be normalized, but must be antisymmetric. 20-4. Suggest the singlet and 3 triplet wave functions of the helium atom with one electron in the 1s orbital and the other electron in the 2s orbital. The wave functions need not be normalized, but must be antisymmetric. 20-5. Explain why the 3 triplet wave functions have the identical energies and are triply degenerate. 20-6. Which of the singlet state of 20-3 and the singlet state of 20-4 has the lower energy? Explain why. 20-7. Which of the singlet and triplet states of 20-4 has the lower energy? Explain why. 20-8. What is the value of ionization energy of the hydrogen atom in eV? 20-9. What is the value of ionization energy of the helium cation in eV? 20-10. Why is the ionization energy of the helium atom much smaller than that of the helium cation (even though both electrons in He are in the 1s orbital just as the sole electron in He+ is)? 20-11. Explain why the Li, Na, and K atoms have relatively low ionization energies. 20-12. Define Hund’s rule and justify it on the basis of the antisymmetry of wave functions.