Hydrogen Atom Exercises - Nebraska Wesleyan University
advertisement

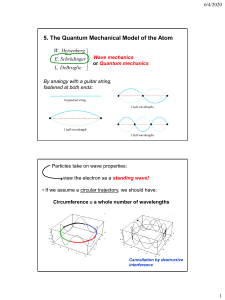
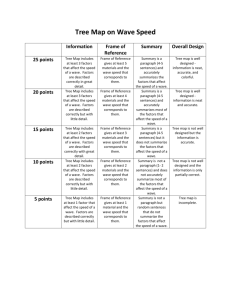
PHYS-251 Intro to Quantum Mech I III4 The Hydrogen Atom 1. NEBRASKA WESLEYAN UNIVERSITY SPRING 2012 – 2013 DUE: FRI 14 APR 2013 Create an animation (a “CAT scan”) of the n = 3, l = 2, m = 0 stationary state of the wave function for the electron bound to a proton to form the H atom. Send the movie to me via email. Answer the following: a) Does this wave function have an imaginary part? Why or why not? b) At the y = 0 plane position, why is the real part positive in some places and negative in others (creating nodal curves similar to those on a vibrating drumhead)? What functions and function values account for these values? c) Is the probability density function a dumbbell or a donut? 2. Create an animation (a “CAT scan”) of the n = 3, l = 2, m = 2 stationary state of the wave function for the electron bound to a proton to form the H atom. Send the movie to me via email. Answer the following: a) Does this wave function have an imaginary part? Why or why not? b) Is the probability density function a dumbbell or a donut? 3. What is a “p orbital?” Try creating a wave function that combines the radial factor with the following linear combination of spherical harmonics: (𝑌1 −1 − 𝑌11 )⁄√2, 𝑖(𝑌1 −1 + 𝑌11 )⁄√2. Note that these are l = 1 states, but that m would be indeterminate. Produce animations showing the wave function and probability density for these. Use n = 2. a) Do these wave functions have imaginary parts? Why or why not? b) Use a chemistry book or online resource to look at the probability density for a p orbital. Do these have the correct appearance? c) What spherical harmonic is a pz orbital? DEAN SIEGLAFF Created: 08 APR 2013 Modified: 1 of 1