Chapter 7-2
advertisement
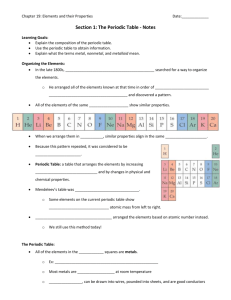
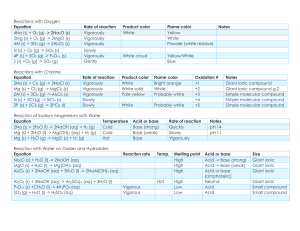
Semester 1 Chemistry Final Study Guide Chapter 1 Be able to distinguish between the chemical and physical properties of matter Classify changes in matter as chemical or physical Explain the gas, liquid and solid phase of matter Distinguish between a mixture and a pure substance Chapter 2 Be able to convert between SI prefixes. For example 2.8 cm= 2.8x10-3km Be familiar with the tables on pg 34, 35, 36 Be able to calculate Mass, density or volume from the equation D=M/V Refresh yourself about the Density of Pennies Lab and its objectives/ procedures Chapter 3 SECTION 3.1 Be able to explain the Law of Conservation of Mass, the Law of Definite Proportions, and the Law of Multiple Proportions Be able to summarize Dalton’s Atomic Theory ( understand which parts of his theory have changed and why) Be able to explain the purpose of the Law of Conservation Lab, observations, discrepancies in data, etc. SECTION 3.2 Be able to summarize the cathode ray experiment and how it led to knowledge that the electron has both mass and charge. Be able to summarize Rutherford’s work the led to current understanding of the nucleus List properties of the protons, neutrons, electrons, and the nucleus. (Table 3-1 on pg 74 would be helpful) Be able to explain why the nucleus is so small and dense (high mass and low volume) it’s due to nuclear forces…see page 74. Define and describe an atom SECTION 3-3 Know how to write both hyphen notation and nuclear symbol for isotopes Be able to calculate mass number, protons, neutrons, and electron for a given isotope. (Ex Sample problem 3-1 pg77) Know the difference between average atomic mass, relative atomic mass, and molar mass. Remember they are all the same number, but have a different use/ meaning. Be able to solve for mass/ moles/ atoms. I will copy the chart on page 82 so make sure that you can use it. Sample problems 3-2 through 3-5 are good practice problems. Remember the best way to prepare for a test is to practice the skill that you will be using on the test… practice problem solving!!! Chapter 22 From 22-1 be able to… Define and relate the terms mass defect and nuclear binding energy Know how to balance a nuclear equation (pg 704 sample and practice problems) Chapter 4 Section 1 Be able to explain the mathematical relationship between the speed, wavelength and frequency of EMR. Be able to solve problems for either frequency of wavelength. Understand that light (EMR) behaves as both a wave and a particle. Know what research that helped support this theory. Understand and be able to explain the significance of the photoelectric effect and the line emission spectrum of hydrogen to the development of the atomic model. Describe the Bohr model of the hydrogen atom and be able to explain why the model was eventually discarded. Chapter 4 Section 2 Be able to describe how the work of DeBroglie helped develop the idea of the quantum model of the atom. Be able to compare and contrast the Bohr model and the quantum model of the atom. Explain how the Heisenberg Uncertainty Principle and the Schrodinger Wave Equation led to the idea of atomic orbitals. List the four quantum numbers and describe what each represents. Relate the number of sub-levels corresponding to each of an atom’s main energy levels, the number of orbitals per sub-level, and the number of orbitals per main energy level. (Table 4-2 pg 104) Chapter 4 Section 3 Be able to list the total number of electrons needed to fully occupy each main energy level. State the Aufbau Principle, the Pauli Exclusion Principle and Hund’s Rule. Understand how each rule governs the configuration of electrons. Describe the electron configuration for the atoms of any element using orbital notation, electron configuration, or noble gas configuration. Be able to determine the number of valence electrons for an element from the electron configuration. Understand that only the highest s and p orbital electrons are valence electrons. So d and f do not have valence electrons. Understand that elements gain or lose electrons in order to become stable like a noble gas. Be able to write the electron for an ion that has lost or gained electrons. Chapter 5-1 Describe the modern periodic table Chapter 5-2 Describe the relationship of electrons in sublevels and the length of each period of the periodic table (table 5-1 pg 128) Locate and name the four blocks of the periodic table. Understand the relationship between group configuration and group number. (table 5-2 pg 137) Describe general properties of elements on the periodic table based on their location (alkali, alkaline-earth, transition metals, non-metals, halogens, noblegases.) Chapter 5-3 Know group and period trends of ionization energy, atomic radii, reactivity, electron affinity, ionic radii, and electronegativity. Chapter 6-1 Define a chemical bond and explain why most atoms form chemical bonds. Describe ionic and covalent bonding Be able to determine if a bond is ionic, polar-covalent, or nonpolar-covalent based on electronegativity differences. Chapter 6-2 State the octet rule Be able to draw Lewis structures for covalently bonded molecules, resonance structures, and polyatomic ions. (Sample problems 6-2 6-4 pgs 170-174 would be good practice Chapter 7-1 Use prefixes to name a binary molecular compound from its formula.Ex., CO2 is carbon dioxide Write the formula of a binary molecular compound given its name Ex., zinc iodide is ZnI2 Determine the formula of an ionic compound formed between two given. Ex., Cobalt (IV) oxide is CoO2 Know common monatomic and polyatomic ions and their charges. (table 7-1 pg 205 and Table 7-2 pg 210) Know the names and formulas of common binary acids and oxyacids (table 75 pg 214) Also covered in chapters 15-16 Chapter 7-2 Assign oxidation numbers of each element in the formula of a chemical compound. (Rules for assigning oxidation numbers are on pg 216 and sample problems on pg 217) Chapter 7-3 Be able to calculate the number of molecules, formula units or ions in a given molar amount of a chemical compound and be able to calculate the percent composition of a given chemical compound (sample problems 7-9 to 7-11 pgs 225- 228 should be good practice.) Chapter 7-4 Determine and empirical formula from either a percentage or a mass composition. (Sample problems 7-12 and 7-13 pgs 230-231) Determine a molecular formula from an empirical formula (Sample problem 7-14 pg 232)